SARS-Cov-2 Vaccination for Children—An Open Issue
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(ACIP) General Best Guidance for Immunization
9. Special Situations Updates Major revisions to this section of the best practices guidance include the timing of intramuscular administration and the timing of clotting factor deficiency replacement. Concurrent Administration of Antimicrobial Agents and Vaccines With a few exceptions, use of an antimicrobial agent does not interfere with the effectiveness of vaccination. Antibacterial agents have no effect on inactivated, recombinant subunit, or polysaccharide vaccines or toxoids. They also have no effect on response to live, attenuated vaccines, except BCG vaccines. Antimicrobial or immunosuppressive agents may interfere with the immune response to BCG and should only be used under medical supervision (for additional information, see www.merck.com/product/usa/pi_circulars/b/bcg/bcg_pi.pdf). Antiviral drugs used for treatment or prophylaxis of influenza virus infections have no effect on the response to inactivated influenza vaccine (2). However, live, attenuated influenza vaccine should not be administered until 48 hours after cessation of therapy with antiviral influenza drugs. If feasible, to avoid possible reduction in vaccine effectiveness, antiviral medication should not be administered for 14 days after LAIV administration (2). If influenza antiviral medications are administered within 2 weeks after receipt of LAIV, the LAIV dose should be repeated 48 or more hours after the last dose of zanamavir or oseltamivir. The LAIV dose should be repeated 5 days after peramivir and 17 days after baloxavir. Alternatively, persons receiving antiviral drugs within the period 2 days before to 14 days after vaccination with LAIV may be revaccinated with another approved vaccine formulation (e.g., IIV or recombinant influenza vaccine). Antiviral drugs active against herpesviruses (e.g., acyclovir or valacyclovir) might reduce the efficacy of vaccines containing live, attenuated varicella zoster virus (i.e., Varivax and ProQuad) (3,4). -

Frequently Asked Questions About Measles Immunizations
Immunization Unit Assessment, Compliance, and Evaluation Group Measles FAQs Q: When did the vaccine for measles become available? A: The first measles vaccines were available in 1963, and were replaced with a much better vaccine in 1968. Q: Do people who received MMR in the 1960s need to have their dose repeated? A: Not necessarily. People who have documentation of receiving a live measles vaccine in the 1960s do not need to be revaccinated. People who were vaccinated with a killed or inactivated measles vaccine, or vaccine of unknown type, should be revaccinated with at least 1 dose of MMR. Q: What kind of vaccine is it? A: The vaccines available in the United States that protect against measles are the MMR and MMRV, which protects against measles, mumps, and rubella or measles, mumps, rubella, and varicella (chickenpox). They are both live attenuated, or weakened, vaccines. Q: Which vaccine can I get, the MMR or the MMRV? A: The MMRV is only approved for people aged 12 months – 12 years. The MMR is approved for anyone 6 months of age and older, but it is routinely recommended for use in people 12 months of age and older. Any dose of MMR received before 12 months of age is not considered valid and should be repeated in 4 weeks or at age 12 months, whichever is later. Q: How is the vaccine given? A: The vaccine is administered in the fatty layer of tissue underneath the skin. Q: Is the measles shot just a one-time dose? A: The measles vaccine is very effective. -

Measles: Chapter 7.1 Chapter 7: Measles Paul A
VPD Surveillance Manual 7 Measles: Chapter 7.1 Chapter 7: Measles Paul A. Gastanaduy, MD, MPH; Susan B. Redd; Nakia S. Clemmons, MPH; Adria D. Lee, MSPH; Carole J. Hickman, PhD; Paul A. Rota, PhD; Manisha Patel, MD, MS I. Disease Description Measles is an acute viral illness caused by a virus in the family paramyxovirus, genus Morbillivirus. Measles is characterized by a prodrome of fever (as high as 105°F) and malaise, cough, coryza, and conjunctivitis, followed by a maculopapular rash.1 The rash spreads from head to trunk to lower extremities. Measles is usually a mild or moderately severe illness. However, measles can result in complications such as pneumonia, encephalitis, and death. Approximately one case of encephalitis2 and two to three deaths may occur for every 1,000 reported measles cases.3 One rare long-term sequelae of measles virus infection is subacute sclerosing panencephalitis (SSPE), a fatal disease of the central nervous system that generally develops 7–10 years after infection. Among persons who contracted measles during the resurgence in the United States (U.S.) in 1989–1991, the risk of SSPE was estimated to be 7–11 cases/100,000 cases of measles.4 The risk of developing SSPE may be higher when measles occurs prior to the second year of life.4 The average incubation period for measles is 11–12 days,5 and the average interval between exposure and rash onset is 14 days, with a range of 7–21 days.1, 6 Persons with measles are usually considered infectious from four days before until four days after onset of rash with the rash onset being considered as day zero. -

Vaccine Information for PARENTS and CAREGIVERS
NATIONAL INSTITUTE FOR COMMUNICABLE DISEASES Division of the National Health Laboratory Service VACCINE INFOR MATION FOR PARENTS & CAREGIVERS First Edition November 2016 Editors-in-Chief Nkengafac Villyen Motaze (MD, MSc, PhD fellow), Melinda Suchard (MBBCh, FCPath (SA), MMed) Edited by: Cheryl Cohen, (MBBCh, FCPath (SA) Micro, DTM&H, MSc (Epi), Phd) Lee Baker, (Dip Pharm) Lucille Blumberg, (MBBCh, MMed (Micro) ID (SA) FFTM (RCPS, Glasgow) DTM&H DOH DCH) Published by: Ideas Wise and Wonderful (IWW) for National Institute for Communicable Diseases (NICD) First Edition: Copyright © 2016 Contributions by: Clement Adu-Gyamfi (BSc Hons, MSc), Jayendrie Thaver (BSc), Kerrigan McCarthy, (MBBCh, FCPath (SA), DTM&H, MPhil (Theol) Kirsten Redman (BSc Hons), Nishi Prabdial-Sing (PhD), Nonhlanhla Mbenenge (MBBCh, MMED) Philippa Hime (midwife), Vania Duxbury (BSc Hons), Wayne Howard (BSc Hons) Acknowledgments: Amayeza, Vaccine Information Centre (http://www.amayeza-info.co.za/) Centre for Communicable Diseases Fact Sheets (http://www.cdc.gov/vaccines/hcp/vis/) World Health Organization Fact Sheets (http://www.who.int/mediacentre/factsheets/en/) National Department of Health, South Africa (http://www.health.gov.za/) Disclaimer This book is intended as an educational tool only. Information may be subject to change as schedules or formulations are updated. Summarized and simplified information is presented in this booklet. For full prescribing information and contraindications for vaccinations, please consult individual package inserts. There has been -
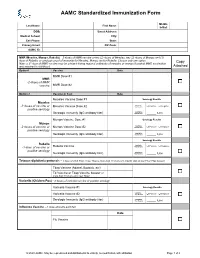
AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

Kids and COVID 19 Vaccination
Kids and COVID 19 Vaccination Q: What COVID vaccine is available for kids right now? A: Pfizer-BioNTech mRNA vaccine was initially approved for individuals to as young as age 16 years old and was recently approved for kids as young as 12 years of age. This is a two shot series, given 3 weeks apart. The dose is the same as that given to adults. Q: When will younger kids be able to get vaccinated? A: Pfizer is currently conducting studies on kids as young as 6 months of age. Moderna is in the process of submitting data on their studies for ages 12-18 yrs and have started the process for studies in younger kids. Many reputable sources suggest that kids as young as 6yrs of age may be eligible for vaccination this fall. Keep checking our website for more information on timeline and details for vaccination in younger kids and infants. Q: How do I get my child vaccinated? A: In North Idaho, there are many locations that offer the Pfizer vaccine. Many local pharmacies have the Pfizer vaccine available (check online or in person to ensure they have Pfizer brand available for those under age 18 yrs). The local Walgreen’s pharmacies are offering Pfizer vaccine and appointments can be scheduled at www.walgreens.com. Other option: www.panhandlehealth.org. This site will allow you to make appointments at the Kootenai county fairgrounds site as well as other sites in adjacent counties. We are not yet offering COVID vaccination at Coeur d’Alene Pediatrics, but hope to be able to do so in the future. -

Statement of David D
THE AMERICAN ASSOCIATION OF IMMUNOLOGISTS Statement of David D. Chaplin, M.D., Ph.D., Chair of the Committee on Public Affairs of The American Association of Immunologists (AAI), Regarding the Importance of Vaccines March 5, 2019 Each year, seasonal outbreaks of influenza cause an estimated 9.3 million to 49 million illnesses, 140,000 to 960,000 hospitalizations, and 12,000 to 79,000 deaths in the United States.1 Other serious infectious diseases also cause occasional outbreaks, often with devastating consequences. A growing outbreak of measles – a dangerous, highly contagious, and potentially lethal disease – is currently spreading in 10 states. It is easily transmitted through the air by coughing and sneezing and by contact with contaminated surfaces. There is no anti-viral treatment for measles. Children younger than five and adults over 20 are at the highest risk of serious complications, including blindness, swelling of the brain, and severe pneumonia.2 Those with compromised immune systems or underlying health conditions who cannot get vaccinated are also at significant risk of complications and death. The spread of many diseases can be limited, or prevented, by available vaccines. For example, efficacy of the existing measles vaccine [measles, mumps, rubella, or “MMR”] is about 97%,3 and its widespread use could prevent measles entirely.4 In other instances, such as with the influenza vaccine, immunization can also lessen the severity of disease, reducing the number of hospitalizations and saving lives.5 AAI is concerned that some parents are choosing not to have their children vaccinated; among the reasons cited is their uncertainty about vaccine safety. -

Adult Immunizations
Guidelines for Clinical Care Ambulatory Immunizations Guideline Team Adult Immunizations Team Leads Susan F Engert, MD, MPH Population: Adults, >18 years old Pediatrics and Communi- cable Diseases Objectives: Implement an evidence-based strategy for routine adult immunizations. Candia B Laughlin, RN, Key Points MS Routine immunizations for adults are: hepatitis A, hepatitis B, herpes zoster, human papilloma virus, Ambulatory Care Nursing Administration influenza, measles, mumps, rubella, meningococcal, pneumococcal, tetanus, diphtheria, pertussis and Team Members varicella. Below is a summary on priority populations, initial vaccination, and revaccination. Margie C Andreae, MD Use combination vaccines whenever possible to increase the coverage rates for vaccine-preventable Pediatrics and Communi- diseases: Tetanus-diphtheria (Td), Tetanus-diphtheria-acellular pertussis (Tdap), Measles-Mumps- cable Diseases Rubella (MMR), hepatitis A-hepatitis B (Twinrix®). Single antigen vaccines have no safety advantage. Mary K.Barry-Bodine, Live virus vaccines (Herpes Zoster, Measles-Mumps-Rubella, Varicella and Live Attenuated Influenza RN, BSN Vaccine) are contraindicated in persons who are pregnant or may become pregnant in the next four Nursing, Health Centers weeks, or who have immunocompromising conditions. If administering multiple live vaccines, give Susan G Blitz, MD, MPH simultaneously or separate them by 4 weeks. Tuberculosis (PPD) skin test should be administered General Medicine before or on the same day as a live virus vaccine or they need to be spaced 4-6 weeks apart. Katie Barwig, RN, MS This guideline follows recommendations of the federal Advisory Committee on Immunization Practices: Nursing Administration These vaccinations should be performed [strength of recommendation] for indicated populations at risk. Sherry L DeLoach, Evidence for each vaccine is based on randomized controlled trials [level of evidence] in general PharmD population and some subgroups, with findings extrapolated to some subgroups. -

(Nitags) and WHO Immunisation-Related Advisory Committees
Summary of recent issues considered by four national immunisation technical advisory groups (NITAGs) and WHO immunisation-related advisory committees Prepared by the National Centre for Immunisation Research & Surveillance (NCIRS) Period of review: 16/05/2019 to 13/09/2019 Contents 1 Advisory Committee on Immunization Practices (ACIP), USA .......................................................... 3 1.1 ACIP meeting: 26-27 June 2019 ....................................................................................................... 3 1.2 Newly published or updated recommendations ............................................................................... 20 1.2.1 Japanese Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunisation Practices.......................................................................................................................... 20 1.2.2 Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practice ..................................................................................................... 20 1.2.3 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2019–20 Influenza Season ............ 21 1.3 New or updated recommendations – not yet published ................................................................... 21 2 Immunisation Advisory Centre (IMAC), New Zealand .................................................................... -

CDC-Measles-Provider-Letter.Pdf
Dear Provider, Due to the current increase in measles cases in the United States (https://www.cdc.gov/mmwr/volumes/68/wr/mm6817e1.htm), the Centers for Disease Control and Prevention has developed the following summary for vaccination of adults against measles with measles, mumps, rubella (MMR) vaccine. Recommendations for vaccination and assessing immunity in adults have not changed since publication of the Advisory Committee on Immunization Practices (ACIP) recommendations for the Prevention of Measles, Rubella, Congenital Rubella syndrome, and Mumps in June 2013. (https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm) WHAT ADULT PROVIDERS NEED TO KNOW: • Providers do not need to actively screen adult patients for measles immunity. This is because of high population immunity and low risk of disease among adults in non-outbreak areas in the U.S. Providers should make sure patients have measles protection before international travel. U.S. residents traveling internationally are at high risk for acquiring measles abroad. They can also transmit measles to susceptible persons, such as infants, when they return home. • If a patient is traveling internationally and measles immunity is unknown, providers should vaccinate, unless there are contraindications. Serologic testing for measles immunity is not recommended. • During outbreaks, providers should consult with local health departments for the most up-to-date recommendations for their community. This may include additional doses of MMR for your patients. Measles is an acute viral illness characterized by a prodrome of fever, cough, coryza, and conjunctivitis, followed by a maculopapular rash. The rash spreads from the head to trunk to the lower extremities. -

Incubation Period of Measles from Exposure to Prodrome ■ Exposure to Rash Onset Averages 11 to 12 Days
Measles Paul Gastanaduy, MD; Penina Haber, MPH; Paul A. Rota, PhD; and Manisha Patel, MD, MS Measles is an acute, viral, infectious disease. References to measles can be found from as early as the 7th century. The Measles disease was described by the Persian physician Rhazes in the ● Acute viral infectious disease 10th century as “more to be dreaded than smallpox.” ● First described in 7th century In 1846, Peter Panum described the incubation period of ● Vaccines first licensed include measles and lifelong immunity after recovery from the disease. measles in 1963, MMR in 1971, John Enders and Thomas Chalmers Peebles isolated the virus in and MMRV in 2005 human and monkey kidney tissue culture in 1954. The first live, ● Infection nearly universal attenuated vaccine (Edmonston B strain) was licensed for use in during childhood in the United States in 1963. In 1971, a combined measles, mumps, prevaccine era and rubella (MMR) vaccine was licensed for use in the United ● Still common and often fatal States. In 2005, a combination measles, mumps, rubella, and in developing countries varicella (MMRV) vaccine was licensed. Before a vaccine was available, infection with measles virus was nearly universal during childhood, and more than 90% of persons were immune due to past infection by age 15 years. Measles is still a common and often fatal disease in developing countries. The World Health Organization estimates there were 142,300 deaths from measles globally in 2018. In the United 13 States, there have been recent outbreaks; the largest occurring in 2019 primarily among people who were not vaccinated. -

Measles Vaccine Our Best Protection
Measles Vaccine: Our Best Protection Measles is a contagious disease that causes high fever, cough, runny nose, and red watery eyes. A rash of tiny red spots breaks out 3-5 days after symptoms begin. It also can lead to infection of the lungs (pneumonia) and brain swelling (encephalitis), which may lead to brain damage or death. There is no treatment for measles, but there is a way to prevent it: the combination measles, mumps, and rubella (MMR) vaccine. It protects you and helps stop the spread of the measles virus to others. People at high risk for severe illness and complications from measles include: • Infants and children less than 5 years of age • Adults greater than 20 years of age • Pregnant women • People with compromised immune systems, such as from leukemia or HIV infection Two doses of MMR vaccine is 97% effective at preventing measles. The vaccine is effective at preventing measles, but it’s not perfect. There is still a small chance of getting measles if you’re vaccinated. Nearly 10 out of 10 people get lasting protection from the vaccine. Number at Risk of Getting Measles Did You Know? Every year, measles is brought into the United States by unvaccinated travelers who 3% get measles while they are in other countries. Anyone who is not protected Vaccinated with MMR against measles is at risk. About 1 in 4 people in the U.S. who get 100% measles will be hospitalized. 1 or 2 out of 1,000 people with measles will Not vaccinated die, even with the best care.