Vaccine Safety: Current Issues and Misunderstandings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(ACIP) General Best Guidance for Immunization
9. Special Situations Updates Major revisions to this section of the best practices guidance include the timing of intramuscular administration and the timing of clotting factor deficiency replacement. Concurrent Administration of Antimicrobial Agents and Vaccines With a few exceptions, use of an antimicrobial agent does not interfere with the effectiveness of vaccination. Antibacterial agents have no effect on inactivated, recombinant subunit, or polysaccharide vaccines or toxoids. They also have no effect on response to live, attenuated vaccines, except BCG vaccines. Antimicrobial or immunosuppressive agents may interfere with the immune response to BCG and should only be used under medical supervision (for additional information, see www.merck.com/product/usa/pi_circulars/b/bcg/bcg_pi.pdf). Antiviral drugs used for treatment or prophylaxis of influenza virus infections have no effect on the response to inactivated influenza vaccine (2). However, live, attenuated influenza vaccine should not be administered until 48 hours after cessation of therapy with antiviral influenza drugs. If feasible, to avoid possible reduction in vaccine effectiveness, antiviral medication should not be administered for 14 days after LAIV administration (2). If influenza antiviral medications are administered within 2 weeks after receipt of LAIV, the LAIV dose should be repeated 48 or more hours after the last dose of zanamavir or oseltamivir. The LAIV dose should be repeated 5 days after peramivir and 17 days after baloxavir. Alternatively, persons receiving antiviral drugs within the period 2 days before to 14 days after vaccination with LAIV may be revaccinated with another approved vaccine formulation (e.g., IIV or recombinant influenza vaccine). Antiviral drugs active against herpesviruses (e.g., acyclovir or valacyclovir) might reduce the efficacy of vaccines containing live, attenuated varicella zoster virus (i.e., Varivax and ProQuad) (3,4). -

Frequently Asked Questions About Measles Immunizations
Immunization Unit Assessment, Compliance, and Evaluation Group Measles FAQs Q: When did the vaccine for measles become available? A: The first measles vaccines were available in 1963, and were replaced with a much better vaccine in 1968. Q: Do people who received MMR in the 1960s need to have their dose repeated? A: Not necessarily. People who have documentation of receiving a live measles vaccine in the 1960s do not need to be revaccinated. People who were vaccinated with a killed or inactivated measles vaccine, or vaccine of unknown type, should be revaccinated with at least 1 dose of MMR. Q: What kind of vaccine is it? A: The vaccines available in the United States that protect against measles are the MMR and MMRV, which protects against measles, mumps, and rubella or measles, mumps, rubella, and varicella (chickenpox). They are both live attenuated, or weakened, vaccines. Q: Which vaccine can I get, the MMR or the MMRV? A: The MMRV is only approved for people aged 12 months – 12 years. The MMR is approved for anyone 6 months of age and older, but it is routinely recommended for use in people 12 months of age and older. Any dose of MMR received before 12 months of age is not considered valid and should be repeated in 4 weeks or at age 12 months, whichever is later. Q: How is the vaccine given? A: The vaccine is administered in the fatty layer of tissue underneath the skin. Q: Is the measles shot just a one-time dose? A: The measles vaccine is very effective. -

Takeda Vaccines Innovation for Global Impact
TAKEDA VACCINES INNOVATION FOR GLOBAL IMPACT CHOO BENG GOH, MD Regional Lead for Medical Affairs Asia, Global Vaccine Business Unit OUR MISSION Develop and deliver innovative vaccines that tackle the toughest problems in public health and improve the lives of people around the world 2 WE HAVE BUILT A GLOBAL VACCINE BUSINESS UPON A STRONG FOUNDATION IN JAPAN Global pivotal Phase 3 PARTNERSHIPS clinical trial of dengue ACQUISITIONS Polio vaccine vaccine candidate initiated: candidate Japan vaccine business Global vaccine business Dengue vaccine 20,100 participants in 8 Bill & Melinda Gates Foundation established established candidate countries in 2 regions Norovirus vaccine Zika vaccine candidate 1946 2012 candidate 2016 U.S. Government‐ BARDA 1947 2010 2014 2018 1st Takeda Multiple vaccine products Partnered with Japan Phase 3 clinical trial results of manufactured vaccine manufactured internally government to develop and dengue vaccine candidate is and marketed in Japan supply pandemic influenza expected in H2 FY18 vaccines for people in Japan 3 THE VACCINE MARKET IS AN ATTRACTIVE PLACE FOR INVESTMENT Vaccine sales growth projected at 7.1% between Durability in sales with limited impact 2017 and 2024, reaching $44.6 billions in 20241 of patent expiry Blockbuster potential in newly launched vaccines Threat of emerging and existing infectious diseases with epidemic potential 1 Evaluate Pharma report 2018 4 OUR STRATEGY Develop vaccines with global BUILD A GLOBAL TACKLE Target the greatest opportunity relevance and business potential PIPELINE -

Measles: Chapter 7.1 Chapter 7: Measles Paul A
VPD Surveillance Manual 7 Measles: Chapter 7.1 Chapter 7: Measles Paul A. Gastanaduy, MD, MPH; Susan B. Redd; Nakia S. Clemmons, MPH; Adria D. Lee, MSPH; Carole J. Hickman, PhD; Paul A. Rota, PhD; Manisha Patel, MD, MS I. Disease Description Measles is an acute viral illness caused by a virus in the family paramyxovirus, genus Morbillivirus. Measles is characterized by a prodrome of fever (as high as 105°F) and malaise, cough, coryza, and conjunctivitis, followed by a maculopapular rash.1 The rash spreads from head to trunk to lower extremities. Measles is usually a mild or moderately severe illness. However, measles can result in complications such as pneumonia, encephalitis, and death. Approximately one case of encephalitis2 and two to three deaths may occur for every 1,000 reported measles cases.3 One rare long-term sequelae of measles virus infection is subacute sclerosing panencephalitis (SSPE), a fatal disease of the central nervous system that generally develops 7–10 years after infection. Among persons who contracted measles during the resurgence in the United States (U.S.) in 1989–1991, the risk of SSPE was estimated to be 7–11 cases/100,000 cases of measles.4 The risk of developing SSPE may be higher when measles occurs prior to the second year of life.4 The average incubation period for measles is 11–12 days,5 and the average interval between exposure and rash onset is 14 days, with a range of 7–21 days.1, 6 Persons with measles are usually considered infectious from four days before until four days after onset of rash with the rash onset being considered as day zero. -

Vaccine Information for PARENTS and CAREGIVERS
NATIONAL INSTITUTE FOR COMMUNICABLE DISEASES Division of the National Health Laboratory Service VACCINE INFOR MATION FOR PARENTS & CAREGIVERS First Edition November 2016 Editors-in-Chief Nkengafac Villyen Motaze (MD, MSc, PhD fellow), Melinda Suchard (MBBCh, FCPath (SA), MMed) Edited by: Cheryl Cohen, (MBBCh, FCPath (SA) Micro, DTM&H, MSc (Epi), Phd) Lee Baker, (Dip Pharm) Lucille Blumberg, (MBBCh, MMed (Micro) ID (SA) FFTM (RCPS, Glasgow) DTM&H DOH DCH) Published by: Ideas Wise and Wonderful (IWW) for National Institute for Communicable Diseases (NICD) First Edition: Copyright © 2016 Contributions by: Clement Adu-Gyamfi (BSc Hons, MSc), Jayendrie Thaver (BSc), Kerrigan McCarthy, (MBBCh, FCPath (SA), DTM&H, MPhil (Theol) Kirsten Redman (BSc Hons), Nishi Prabdial-Sing (PhD), Nonhlanhla Mbenenge (MBBCh, MMED) Philippa Hime (midwife), Vania Duxbury (BSc Hons), Wayne Howard (BSc Hons) Acknowledgments: Amayeza, Vaccine Information Centre (http://www.amayeza-info.co.za/) Centre for Communicable Diseases Fact Sheets (http://www.cdc.gov/vaccines/hcp/vis/) World Health Organization Fact Sheets (http://www.who.int/mediacentre/factsheets/en/) National Department of Health, South Africa (http://www.health.gov.za/) Disclaimer This book is intended as an educational tool only. Information may be subject to change as schedules or formulations are updated. Summarized and simplified information is presented in this booklet. For full prescribing information and contraindications for vaccinations, please consult individual package inserts. There has been -

Thimerosal in Vaccines—An Interim Report to Clinicians
AMERICAN ACADEMY OF PEDIATRICS Committee on Infectious Diseases and Committee on Environmental Health Thimerosal in Vaccines—An Interim Report to Clinicians ABBREVIATIONS. AAP, American Academy of Pediatrics; and for preventing bacterial contamination, particu- USPHS, US Public Health Service; FDA, Food and Drug Admin- larly in opened multidose containers. Some but not istration; IPV, inactivated polio vaccine; OPV, oral polio vaccine; all of the vaccines recommended routinely for chil- DTP, diphtheria-tetanus-pertussis (vaccine); Hib, Haemophilus in- dren in the United States contain thimerosal.1 fluenzae type b (vaccine); EPA, Environmental Protection Agency; ATSDR, Agency for Toxic Substances and Disease Registry; Thimerosal contains 49.6% mercury by weight and is HBsAg, hepatitis B surface antigen; HBIG, hepatitis B immune metabolized to ethyl mercury and thiosalicylate. globulin. Data are limited regarding potential differences in toxicity between ethyl mercury and methyl mercury. n July 7, 1999, the American Academy of Both forms of organic mercury are associated with Pediatrics (AAP) issued with the US Public neurotoxicity in high doses, and definitive data re- Health Service (USPHS) a joint statement garding the doses at which developmental effects O occur in infants are not available. When vaccines alerting clinicians and the public of concern about thimerosal, a mercury-containing preservative used containing thimerosal have been administered in the in some vaccines. That statement was disseminated recommended doses, hypersensitivity has been widely, including on the AAP members e-mail list, noted, but no other harmful effects have been report- 2 Massive overdoses from inappropriate use of and was posted on the AAP web site since July 7, ed. -

HPV Vaccine Update
Dengue modeling Andrea Vicari Comprehensive Family Immunization Unit Family, Gender and Life Course Department Outline • Framing modeling within policy cycle • Purposes of infectious disease modeling • Overview of dengue modeling • Final considerations Five stages of a policy cycle Agenda setting • Problem recognition Policy formulation • Proposal of solution Decision-making • Choice of solution Policy implementation • Putting solution into effect Policy evaluation • Monitoring results Howlett & Ramesh, Studying public policy: Policy cycles and policy subsystems, 1995 Identify the decision situation and A decision- understand objectives analysis process Identify alternatives flowchart Decompose and model the problem: 1. Model of problem structure 2. Model of uncertainty 3. Model of preferences Choose best alternatives Sensitivity analysis Is further YES analysis necessary? NO Implement chosen alternative Clemen and Reilly, Making hard decisions, 2002. Main purposes of infectious disease modeling • To understand fundamental driving forces of disease ecology and epidemiology • To measure epidemiological parameters that cannot be directly measured with field or laboratory data • To make predictions of future disease incidence under specified conditions • To forecast impact of different prevention/control measures and their combination Adapted from: WHO-VMI Dengue Vaccine Modeling Group, PLoS Negl Trop Dis 2012, 6:e1450 Classical Ross-Macdonald model for malaria transmission (1) Classical Ross-Macdonald model for malaria transmission (2) m -
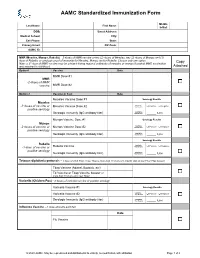
AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

Dengue Vaccine: Global Development Update
REVIEW ARTICLE Asian Pacific Journal of Allergy and Immunology Dengue vaccine: Global development update Eakachai Prompetchara,1,2,3 Chutitorn Ketloy,3,4 Stephen J. Thomas,5 Kiat Ruxrungtham4,6 Abstract The first licensed dengue vaccine, CYD-TDV (Dengvaxia®), has received regulatory approval in a number of countries. However, this vaccine has some limitations. Its efficacy against DENV2 was consistently lower than other serotypes. Protective efficacy also depended on prior dengue sero-status of the vaccinees. Lower efficacy was observed in children with < 9 years old and dengue-naïve individuals. More importantly, risk of hospitalization and severe dengue was in- creased in the youngest vaccine recipients (2-5 years) compared to controls. Thus, the quest of a better vaccine candi- date continues. There are two live-attenuated vaccine candidates currently testing in phase III trial including DENVax, developed by US CDC and Inviragen (now licensed to Takeda) and TV003/TV005, constructed by US NIAID. In addi- tion, there are several phase I–II as well as preclinical phase studies evaluating vaccines for safety and immunogenicity, this include other live-attenuated platform/strategy, purified-inactivated viruses formulated with adjuvants, DNA vac- cine, subunit vaccine, viral vector and also heterologous prime/boost strategies. The major difficulties of dengue vaccine development are included the lack of the best animal model, various immune status of individual especially in endemic areas and clear cut off of protective immunity. Several research and development efforts are ongoing to find a better effec- tive and accessible dengue vaccine for people needed. Key words: Dengue vaccine, Live-attenuated virus, Inactivated virus, DNA vaccine, Subunit vaccine, Heterologous prime- boost From: is the result of worldwide transportation and travel, increas- 1 Department of Biochemistry and Microbiology, ing urbanization, as well as climate change which support Faculty of Pharmaceutical Sciences, Chulalongkorn University, 3 Bangkok, Thailand the spreading of Aedes mosquitoes. -

Needle Tips Fall/Winter 1998-1999
Volume 8 - Number 2 NEEDLE TIPS Fall/Winter 1998-1999 & the Hepatitis B Coalition News Published by the Immunization Action Coalition for individuals and organizations concerned about vaccine-preventable diseases. Holy contraindications, Robin! That person was coughing! WHAT'S ON THE INSIDE: Why did you vaccinate him? Ask the Experts CDC’s immunization expert Dr. William Atkinson answers your questions ......................... 1 CDC’s hepatitis experts, Harold Margolis, MD, and Linda Moyer, RN, answer hepatitis A and B questions .................................................................................................. 17 What’s New? Vaccinate, don’t vacillate! by Walter A. Orenstein, MD, Centers for Disease Control ........... 3 Vaccine highlights: new vaccines and new recommendations .............................................. 4 ? ? ? It’s federal law! by Neal Halsey, MD, Institute for Vaccine Safety ....................................... 6 What’s your state doing? hepatitis B laws, pneumococcal, influenza rates, etc. .................... 9 Unprotected people...five varicella-related deaths ................................................................ 13 Photocopy these materials! Holy missed opportunities, Batman! Polio Vaccine Information Statement (VIS) ......................................................................... 7 A mild illness is NOT a reason to NEW! Vaccinations for adults with hepatitis C virus infection ............................................. 10 withhold vaccinations. You’d know this, Revised! -

Vaccine Refusal, Mandatory Immunization, and the Risks of Vaccine-Preventable Diseases
The new england journal of medicine special article Vaccine Refusal, Mandatory Immunization, and the Risks of Vaccine-Preventable Diseases Saad B. Omer, M.B., B.S., Ph.D., M.P.H., Daniel A. Salmon, Ph.D., M.P.H., Walter A. Orenstein, M.D., M. Patricia deHart, Sc.D., and Neal Halsey, M.D. Abstract Vaccines are among the most effective prevention tools available to clinicians. How- From the Hubert Department of Global ever, the success of an immunization program depends on high rates of acceptance Health, Rollins School of Public Health (S.B.O.), and the Emory Vaccine Center and coverage. There is evidence of an increase in vaccine refusal in the United States (S.B.O., W.A.O.), Emory University, At- and of geographic clustering of refusals that results in outbreaks. Children with lanta; the Department of International exemptions from school immunization requirements (a measure of vaccine refusal) Health (S.B.O., D.A.S., N.H.) and the In- stitute for Vaccine Safety (N.H.), Johns are at increased risk for measles and pertussis and can infect others who are too Hopkins Bloomberg School of Public young to be vaccinated, cannot be vaccinated for medical reasons, or were vacci- Health, Baltimore; the National Vaccine nated but did not have a sufficient immunologic response. Clinicians can play a Program Office, Department of Health and Human Services, Washington, DC crucial role in parental decision making. Health care providers are cited as the most (D.A.S.); and Maternal and Child Health frequent source of immunization information by parents, including parents of un- Assessment, Washington State Depart- vaccinated children. -

Kids and COVID 19 Vaccination
Kids and COVID 19 Vaccination Q: What COVID vaccine is available for kids right now? A: Pfizer-BioNTech mRNA vaccine was initially approved for individuals to as young as age 16 years old and was recently approved for kids as young as 12 years of age. This is a two shot series, given 3 weeks apart. The dose is the same as that given to adults. Q: When will younger kids be able to get vaccinated? A: Pfizer is currently conducting studies on kids as young as 6 months of age. Moderna is in the process of submitting data on their studies for ages 12-18 yrs and have started the process for studies in younger kids. Many reputable sources suggest that kids as young as 6yrs of age may be eligible for vaccination this fall. Keep checking our website for more information on timeline and details for vaccination in younger kids and infants. Q: How do I get my child vaccinated? A: In North Idaho, there are many locations that offer the Pfizer vaccine. Many local pharmacies have the Pfizer vaccine available (check online or in person to ensure they have Pfizer brand available for those under age 18 yrs). The local Walgreen’s pharmacies are offering Pfizer vaccine and appointments can be scheduled at www.walgreens.com. Other option: www.panhandlehealth.org. This site will allow you to make appointments at the Kootenai county fairgrounds site as well as other sites in adjacent counties. We are not yet offering COVID vaccination at Coeur d’Alene Pediatrics, but hope to be able to do so in the future.