Emergency Department Triage of Traumatic Head Injury Using Brain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Management of Retained Hemothorax in Blunt Head and Chest Trauma
World J Surg https://doi.org/10.1007/s00268-017-4420-x ORIGINAL SCIENTIFIC REPORT Early Management of Retained Hemothorax in Blunt Head and Chest Trauma 1,2 1,8 1,7 1 Fong-Dee Huang • Wen-Bin Yeh • Sheng-Shih Chen • Yuan-Yuarn Liu • 1 1,3,6 4,5 I-Yin Lu • Yi-Pin Chou • Tzu-Chin Wu Ó The Author(s) 2018. This article is an open access publication Abstract Background Major blunt chest injury usually leads to the development of retained hemothorax and pneumothorax, and needs further intervention. However, since blunt chest injury may be combined with blunt head injury that typically requires patient observation for 3–4 days, other critical surgical interventions may be delayed. The purpose of this study is to analyze the outcomes of head injury patients who received early, versus delayed thoracic surgeries. Materials and methods From May 2005 to February 2012, 61 patients with major blunt injuries to the chest and head were prospectively enrolled. These patients had an intracranial hemorrhage without indications of craniotomy. All the patients received video-assisted thoracoscopic surgery (VATS) due to retained hemothorax or pneumothorax. Patients were divided into two groups according to the time from trauma to operation, this being within 4 days for Group 1 and more than 4 days for Group 2. The clinical outcomes included hospital length of stay (LOS), intensive care unit (ICU) LOS, infection rates, and the time period of ventilator use and chest tube intubation. Result All demographics, including age, gender, and trauma severity between the two groups showed no statistical differences. -

NIH Public Access Author Manuscript J Neuropathol Exp Neurol
NIH Public Access Author Manuscript J Neuropathol Exp Neurol. Author manuscript; available in PMC 2010 September 24. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: J Neuropathol Exp Neurol. 2009 July ; 68(7): 709±735. doi:10.1097/NEN.0b013e3181a9d503. Chronic Traumatic Encephalopathy in Athletes: Progressive Tauopathy following Repetitive Head Injury Ann C. McKee, MD1,2,3,4, Robert C. Cantu, MD3,5,6,7, Christopher J. Nowinski, AB3,5, E. Tessa Hedley-Whyte, MD8, Brandon E. Gavett, PhD1, Andrew E. Budson, MD1,4, Veronica E. Santini, MD1, Hyo-Soon Lee, MD1, Caroline A. Kubilus1,3, and Robert A. Stern, PhD1,3 1 Department of Neurology, Boston University School of Medicine, Boston, Massachusetts 2 Department of Pathology, Boston University School of Medicine, Boston, Massachusetts 3 Center for the Study of Traumatic Encephalopathy, Boston University School of Medicine, Boston, Massachusetts 4 Geriatric Research Education Clinical Center, Bedford Veterans Administration Medical Center, Bedford, Massachusetts 5 Sports Legacy Institute, Waltham, MA 6 Department of Neurosurgery, Boston University School of Medicine, Boston, Massachusetts 7 Department of Neurosurgery, Emerson Hospital, Concord, MA 8 CS Kubik Laboratory for Neuropathology, Department of Pathology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts Abstract Since the 1920s, it has been known that the repetitive brain trauma associated with boxing may produce a progressive neurological deterioration, originally termed “dementia pugilistica” and more recently, chronic traumatic encephalopathy (CTE). We review the 47 cases of neuropathologically verified CTE recorded in the literature and document the detailed findings of CTE in 3 professional athletes: one football player and 2 boxers. -

Mass/Multiple Casualty Triage
9.1 MASS/MULTIPLE CASUALTY TRIAGE PURPOSE · The goal of the mass/multiple Casualty Triage protocol is to prepare for a unified, coordinated, and immediate EMS mutual aid response by prehospital and hospital agencies to effectively expedite the emergency management of the victims of any type of Mass Casualty Incident (MCI). · Successful management of any MCI depends upon the effective cooperation, organization, and planning among health care professionals, hospital administrators and out-of-hospital EMS agencies, state and local government representatives, and individuals and/or organizations associated with disaster-related support agencies. · Adoption of Model Uniform Core Criteria (MUCC). DEFINITIONS Multiple Casualty Situations · The number of patients and the severity of the injuries do not exceed the ability of the provider to render care. Patients with life-threatening injuries are treated first. Mass Casualty Incidents · The number of patients and the severity of the injuries exceed the capability of the provider, and patients sustaining major injuries who have the greatest chance of survival with the least expenditure of time, equipment, supplies, and personnel are managed first. H a z GENERAL CONSIDERATIONS m Initial assessment to include the following: a t · Location of incident. & · Type of incident. M · Any hazards. C · Approximate number of victims. I · Type of assistance required. 9 . 1 COMMUNICATION · Within the scope of a Mass Casualty Incident, the EMS provider may, within the limits of their scope of practice, perform necessary ALS procedures, that under normal circumstances would require a direct physician’s order. · These procedures shall be the minimum necessary to prevent the loss of life or the critical deterioration of a patient’s condition. -
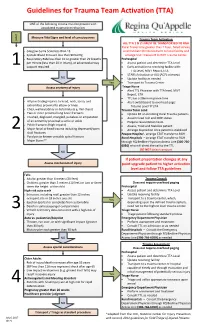
Guidelines for Trauma Team Activation (TTA)
Guidelines for Trauma Team Activation (TTA) ONE of the following criteria must be present with associated traumatic mechanism L e v e Measure Vital Signs and level of consciousness l Trauma Team Activation ALL TTA 1 & 2's MUST BE TRANSPORTED TO RGH Rural Travel time greater than 1 hour, failed airway · Glasgow Coma Scale less than 13 or immediate life threat divert to local facility and · Systolic Blood Pressure less than 90mmHg arrange STAT transport to RGH Trauma Center · Respiratory Rate less than 10 or greater then 29 breaths Prehospital per minute (less than 20 in infant), or advanced airway · Assess patient and determine TTA Level 1 support required · Early activation to receiving facility with: TTA Level, MIVT Report, ETA · STARS Activation or ALS (ACP) intercept NO · Update facility as needed Yes · Transport to Trauma Center Assess anatomy of injury Triage Nurse · Alert TTL Physician with TTA level, MIVT Report, ETA · TTL has a 20min response time · All penetrating injuries to head, neck, torso, and · Alert switchboard to overhead page: extremities proximal to elbow or knee Trauma Level ‘#’ ETA · Chest wall instability or deformity (e.g. flail chest) Trauma Team Lead · Two or more proximal long-bone fractures · Update ER on incoming Rural Trauma patients · Crushed, degloved, mangled, pulseless or amputation · Assume lead role and MRP status of an extremity proximal to wrist or ankle · Prepare resuscitation team · Pelvic fractures (high impact) · Assess, Treat and Stabilize patient 2 · Major facial or head trauma including depressed/open -

MASS CASUALTY TRAUMA TRIAGE PARADIGMS and PITFALLS July 2019
1 Mass Casualty Trauma Triage - Paradigms and Pitfalls EXECUTIVE SUMMARY Emergency medical services (EMS) providers arrive on the scene of a mass casualty incident (MCI) and implement triage, moving green patients to a single area and grouping red and yellow patients using triage tape or tags. Patients are then transported to local hospitals according to their priority group. Tagged patients arrive at the hospital and are assessed and treated according to their priority. Though this triage process may not exactly describe your agency’s system, this traditional approach to MCIs is the model that has been used to train American EMS As a nation, we’ve got a lot providers for decades. Unfortunately—especially in of trailers with backboards mass violence incidents involving patients with time- and colored tape out there critical injuries and ongoing threats to responders and patients—this model may not be feasible and may result and that’s not what the focus in mis-triage and avoidable, outcome-altering delays of mass casualty response is in care. Further, many hospitals have not trained or about anymore. exercised triage or re-triage of exceedingly large numbers of patients, nor practiced a formalized secondary triage Dr. Edward Racht process that prioritizes patients for operative intervention American Medical Response or transfer to other facilities. The focus of this paper is to alert EMS medical directors and EMS systems planners and hospital emergency planners to key differences between “conventional” MCIs and mass violence events when: • the scene is dynamic, • the number of patients far exceeds usual resources; and • usual triage and treatment paradigms may fail. -

Guidelines for BLS/ALS Medical Providers Current As of March 2019
Tactical Emergency Casualty Care (TECC) Guidelines for BLS/ALS Medical Providers Current as of March 2019 DIRECT THREAT CARE (DTC) / HOT ZONE Guidelines: 1. Mitigate any immediate threat and move to a safer position (e.g. initiate fire attack, coordinated ventilation, move to safe haven, evacuate from an impending structural collapse, etc). Recognize that threats are dynamic and may be ongoing, requiring continuous threat assessments. 2. Direct the injured first responder to stay engaged in the operation if able and appropriate. 3. Move patient to a safer position: a. Instruct the alert, capable patient to move to a safer position and apply self-aid. b. If the patient is responsive but is injured to the point that he/she cannot move, a rescue plan should be devised. c. If a patient is unresponsive, weigh the risks and benefits of an immediate rescue attempt in terms of manpower and likelihood of success. Remote medical assessment techniques should be considered to identify patients who are dead or have non-survivable wounds. 4. Stop life threatening external hemorrhage if present and reasonable depending on the immediate threat, severity of the bleeding and the evacuation distance to safety. Consider moving to safety prior to application of the tourniquet if the situation warrants. a. Apply direct pressure to wound, or direct capable patient to apply direct pressure to own wound and/or own effective tourniquet. b. Tourniquet application: i. Apply the tourniquet as high on the limb as possible, including over the clothing if present. ii. Tighten until cessation of bleeding and move to safety. -

Early Post-Traumatic Pulmonary-Embolism in Patients Requiring ICU Admission: More Complicated Than We Think!
3854 Editorial Early post-traumatic pulmonary-embolism in patients requiring ICU admission: more complicated than we think! Mabrouk Bahloul1, Mariem Dlela1, Nadia Khlaf Bouaziz2, Olfa Turki1, Hedi Chelly1, Mounir Bouaziz1 1Department of Intensive Care, Habib Bourguiba University Hospital, Sfax, Tunisia; 2Centre Intermédiaire, Rte El MATAR Km 4, Sfax, Tunisia Correspondence to: Professor Mabrouk Bahloul. Department of Intensive Care, Habib Bourguiba University Hospital, 3029 Sfax, Tunisia. Email: [email protected]. Submitted Jun 10, 2018. Accepted for publication Sep 12, 2018. doi: 10.21037/jtd.2018.09.49 View this article at: http://dx.doi.org/10.21037/jtd.2018.09.49 We read with interest the article entitled “Prevalence and traumatic thromboembolism in patients who are requiring main determinants of early post-traumatic thromboembolism an ICU admission. Nevertheless, this study as mentioned in patients requiring ICU admission” (1). In this retrospective by the authors suffers from many limitations. In fact, the study, Kazemi Darabadi et al. (1) had included to their small sample size, and also the retrospective design of database, the records of 240 trauma-patients requiring the study lead to an increased probability of some bias ICU admission, with a confirmed diagnosis of pulmonary when collecting data. In fact, in this study (1), PE was embolism (PE). The patients were categorized as subjects not screened on a daily basis. As a consequence, the late with an early PE (≤3 days) and those with a late PE (>3 days). stage PE may only be a delayed diagnosis. For instance, According to their analysis, 48.5% of the patients suffering a patient who develops with PE on day 2 but without any from PE had developed this complication within 72 h symptom(s); then he is accidentally diagnosed with PE on following a trauma event and/or after ICU admission. -

The Fund at a Glance
CytoSorbentsTM Trauma and Crush Injury TRAUMA AND CRUSH INJURIES ARE CURRENTLY LEADING CAUSES OF DEATH. Trauma and crush injuries occur frequently and from multiple sources. For example, automobile or motorcycle accidents, natural disasters, and acts of criminal violence or terrorism can all yield mild to severe trauma and crush injuries. The breadth of life-threatening conditions from trauma-induced injuries, such as lung and bowel rupture, closed head injury, crush injury and rhabdomyolysis, traumatic fractures, penetrating wound injury, and limb loss, creates a very challenging environment for clinicians to employ effective treatment strategies. Better therapies are needed to reduce the mortality in patients with severe trauma injury, reduce the risk of organ failure, reduce complications (e.g. rhabdomyolysis and infection), and decrease hospital stay length. CYTOKINE STORM AND RHABDOMYOLYSIS IN TRAUMA CAN CAUSE ORGAN FAILURE AND DEATH Trauma is a well-known trigger of the immune response, and can result in the massive, uncontrolled production of cytokines, or “cytokine storm.” Cytokines are small proteins that normally help stimulate and regulate the immune system to fight infection or injury. However, in trauma, cytokine storm can be toxic, leading to a massive systemic inflammatory response syndrome (SIRS) and a cascade of events that can kill cells and damage organs, leading to organ failure and often death. Cytokine storm exacerbates physical trauma in many ways. For example, trauma can cause: Hemorrhagic shock due to blood loss while cytokine storm causes capillary leak and intravascular volume loss, and the induction of nitric oxide that can lead to myocardial depression and peripheral vasodilation, all of which exacerbate shock Severe limb injuries that often require a tourniquet. -

Traumatic Brain Injury (TBI)
Traumatic Brain Injury (TBI) Carol A. Waldmann, MD raumatic brain injury (TBI), caused either by blunt force or acceleration/ deceleration forces, is common in the general population. Homeless persons Tare at particularly high risk of head trauma and adverse outcomes to TBI. Even mild traumatic brain injury can lead to persistent symptoms including cognitive, physical, and behavioral problems. It is important to understand brain injury in the homeless population so that appropriate referrals to specialists and supportive services can be made. Understanding the symptoms and syndromes caused by brain injury sheds light on some of the difficult behavior observed in some homeless persons. This understanding can help clinicians facilitate and guide the care of these individuals. Prevalence and Distribution recover fully, but up to 15% of patients diagnosed TBI and Mood Every year in the USA, approximately 1.5 with MTBI by a physician experience persistent Swings. million people sustain traumatic brain injury disabling problems. Up to 75% of brain injuries This man suffered (TBI), 230,000 people are hospitalized due to TBI are classified as MTBI. These injuries cost the US a gunshot wound and survive, over 50,000 people die from TBI, and almost $17 billion per year. The groups most at risk to the head and many subsequent more than 1 million people are treated in emergency for TBI are those aged 15-24 years and those aged traumatic brain rooms for TBI. In persons under the age of 45 years, 65 years and older. Men are twice as likely to sustain injuries while TBI is the leading cause of death. -

A Uniform Triage Scale in Emergency Medicine Information Paper
A Uniform Triage Scale in Emergency Medicine Information Paper Triage: sorting, sifting (Webster’s New Collegiate Dictionary) from the French verb trier- “to sort.” Triage has long been considered a simple frontline sorting mechanism in hospital-based emergency departments (EDs). However, evolution in the practice of emergency medicine during the past two decades necessitates a change in how this entry point process is performed and utilized. Many triage systems are in use in the US, but there is no uniform triage scale that would facilitate the development of operational standards in EDs. A nationally standardized triage scale would provide an analytic basis for determining whether the health care system provides safe access to emergency care based on design, resources, and utilization. The performance of EDs could be compared based on case mix and acuity, and expected standards for facilities could be defined. Planners and policy makers would have the tools and the data needed to make rational improvements in the health care delivery system. This paper on triage will acquaint the reader with the history of triage, and provide an overview of the Australian and Canadian systems which are already in use on a national level. The reliability of triage is addressed, and the Canadian and Australian scales are compared. Future implications for a national triage scale are described, along with the goals and benefits of triage development. While there is some controversy about potential liability issues, the many advantages of a national triage scale appear to outweigh any potential disadvantages. History of triage The first medical application of triage occurred on the French battlefield where sorting the victims determined who would be left behind. -

Severe Localized Scapular Pain After a Strenuous Weight-Lifting Session
CASE REPORT Severe localized scapular Editor’s key points With the rising popularity of ultra- pain after a strenuous distance endurance events, strength and conditioning programs, and obstacle course races, exertional weight-lifting session rhabdomyolysis (ER) has become increasingly common in sporting Rosamond E. Lougheed Simpson MD MSc CCFP(SEM) DipSportMed communities. Steven R. Joseph MD MA CCFP(SEM) DipSportMed Lisa Fischer MD CCFP(SEM) FCFP DipSportMed Exertional rhabdomyolysis is often characterized by the classic triad habdomyolysis is a medical condition whereby the intracellular con- of generalized weakness, myalgia, tents from damaged skeletal muscle tissues are released into the blood, and myoglobinuria; however, it is critical to recognize that many cases causing myriad clinical symptoms and outcomes. These can range will not present with all 3 of these Rfrom muscle pain to compartment syndrome, end-organ failure, and death.1-3 criteria. For the male patient in this While the triggers of rhabdomyolysis are numerous, physical exertion as a report, severe myalgia was his only presenting symptom from the triad. causal factor has been receiving increasing media attention recently.4-7 The incidence of exertional rhabdomyolysis (ER) has been challenging The ability to recognize ER, stratify 8 patients into low- and high-risk to estimate, as many cases are likely underrecognized. Current incidence categories, and understand how estimates range from 22.2 to 29.9 per 100 000 patients a year.9,10 As ultra- risk affects patients’ return to play distance endurance events, strength and conditioning programs (eg, CrossFit), can help family physicians make treatment, referral, and return- and obstacle course races have become wildly popular with the superfit to-play decisions with increased and weekend warriors alike, ER is being increasingly recognized as common confidence. -

Redefining the Trauma Triage Matrix
journalofsurgicalresearch july 2020 (251) 195e201 Available online at www.sciencedirect.com ScienceDirect journal homepage: www.JournalofSurgicalResearch.com Redefining the Trauma Triage Matrix: The Role of Emergent Interventions Rachel S. Morris, MD,a,* Nicholas J. Davis, MD,b Amy Koestner, MSN,c Lena M. Napolitano, MD,d Mark R. Hemmila, MD,d and Christopher J. Tignanelli, MDa,b,e a Department of Surgery, University of Minnesota, Minneapolis, Minnesota b Department of Surgery, North Memorial Medical Center, Robbinsdale, Minnesota c Department of Surgery, Spectrum Health - Butterworth Hospital, Grand Rapids, Michigan d Department of Surgery, University of Michigan, Ann Arbor, Michigan e Institute for Health Informatics, University of Minnesota, Minneapolis, Minnesota article info abstract Article history: Background: A tiered trauma team activation (TTA) system aims to allocate resources pro- Received 10 July 2019 portional to the patient’s need based upon injury burden. The current metrics used to Received in revised form evaluate appropriateness of TTA are the trauma triage matrix (TTM), need for trauma 22 October 2019 intervention (NFTI), and secondary triage assessment tool (STAT). Accepted 2 November 2019 Materials and methods: In this retrospective study, we compared the effectiveness of the Available online xxx need for an emergent intervention within 6 h (NEI-6) with existing definitions. Data from the Michigan Trauma Quality Improvement Program was utilized. The dataset contains Keywords: information from 31 level 1 and 2 trauma centers from 2011 to 2017. Inclusion criteria were: Activation adult patients (16 y) and ISS 5. Trauma Results: 73,818 patients were included in the study. Thirty percentage of trauma patients Triage met criteria for STAT, 21% for NFTI, 20% for TTM, and 13% for NEI-6.