Sclera and Retina Suturing Techniques 9 Kirk H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors
pharmaceutics Article Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors Hyeong Min Kim 1,†, Hyounkoo Han 2,†, Hye Kyoung Hong 1, Ji Hyun Park 1, Kyu Hyung Park 1, Hyuncheol Kim 2,* and Se Joon Woo 1,* 1 Department of Ophthalmology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam 13620, Korea; [email protected] (H.M.K.); [email protected] (H.K.H.); [email protected] (J.H.P.); [email protected] (K.H.P.) 2 Department of Chemical and Biomolecular Engineering, Sogang University, Seoul 04107, Korea; [email protected] * Correspondence: [email protected] (H.K.); [email protected] (S.J.W.); Tel.: +82-2-705-8922 (H.K.); +82-31-787-7377 (S.J.W.); Fax: +82-2-3273-0331 (H.K.); +82-31-787-4057 (S.J.W.) † These authors contributed equally to this work. Abstract: In this study, Retina-RPE-Choroid-Sclera (RCS) and RPE-Choroid-Sclera (CS) were prepared by scraping them off neural retina, and using the Ussing chamber we measured the average time– concentration values in the acceptor chamber across five isolated rabbit tissues for each drug molecule. We determined the outward direction permeability of the RCS and CS and calculated the neural retina permeability. The permeability coefficients of RCS and CS were as follows: ganciclovir, 13.78 ± 5.82 and 23.22 ± 9.74; brimonidine, 15.34 ± 7.64 and 31.56 ± 12.46; bevacizumab, 0.0136 ± 0.0059 and 0.0612 ± 0.0264 (×10−6 cm/s). -

How the Eye Works
HOW THE EYE WORKS The Eyes & Vision Our ability to "see" starts when light reflects off an object and enters the eye. As it enters the eye, the light is unfocused. The first step in seeing is to focus the light rays onto the retina, which is the light sensitive layer found inside the eye. Once the light is focused, it stimulates cells to send millions of electrochemical impulses along the optic nerve to the brain. The portion of the brain at the back of the head interprets the impulses, enabling us to see the object. The Refraction of Light by the Eye Light entering the eye is first bent, or refracted, by the cornea -- the clear window on the outer front surface of the eyeball. The cornea provides most of the eye's optical power or light- bending ability. After the light passes through the cornea, it is bent again -- to a more finely adjusted focus -- by the crystalline lens inside the eye. The lens focuses the light on the retina. This is achieved by the ciliary muscles in the eye. They change the shape of the lens, bending or flattening it to focus the light rays on the retina. This adjustment in the lens is necessary for bringing near and far objects into focus. The process of bending light to produce a focused image on the retina is called "refraction". Ideally, the light is "refracted" in such a manner that the rays are focused into a precise image on the retina. Many vision problems occur because of an error in how our eyes refract light. -

Retinal Anatomy and Histology
1 Q Retinal Anatomy and Histology What is the difference between the retina and the neurosensory retina? 2 Q/A Retinal Anatomy and Histology What is the difference between the retina and the neurosensory retina? While often used interchangeably (including, on occasion, in this slide-set), these are technically not synonyms. The term neurosensory retina refers to the neural lining on the inside of the eye, whereas the term retina refers to this neural lining along with the retinal pigmentthree epithelium words (RPE). 3 A Retinal Anatomy and Histology What is the difference between the retina and the neurosensory retina? While often used interchangeably (including, on occasion, in this slide-set), these are technically not synonyms. The term neurosensory retina refers to the neural lining on the inside of the eye, whereas the term retina refers to this neural lining along with the retinal pigment epithelium (RPE). 4 Q Retinal Anatomy and Histology What is the difference between the retina and the neurosensory retina? While often used interchangeably (including, on occasion, in this slide-set), these are technically not synonyms. The term neurosensory retina refers to the neural lining on the inside of the eye, whereas the term retina refers to this neural lining along with the retinal pigment epithelium (RPE). The neurosensory retina contains three classes of cells—what are they? There are five types of neural elements—what are they? What are the three types of glial cells? The two vascular cell types? --? ----PRs ----Bipolar cells ----Ganglion cells ----Amacrine cells ----Horizontal cells --? ----Müeller cells ----Astrocytes ----Microglia --? ----Endothelial cells ----Pericytes 5 A Retinal Anatomy and Histology What is the difference between the retina and the neurosensory retina? While often used interchangeably (including, on occasion, in this slide-set), these are technically not synonyms. -

Anatomy and Physiology of the Afferent Visual System
Handbook of Clinical Neurology, Vol. 102 (3rd series) Neuro-ophthalmology C. Kennard and R.J. Leigh, Editors # 2011 Elsevier B.V. All rights reserved Chapter 1 Anatomy and physiology of the afferent visual system SASHANK PRASAD 1* AND STEVEN L. GALETTA 2 1Division of Neuro-ophthalmology, Department of Neurology, Brigham and Womens Hospital, Harvard Medical School, Boston, MA, USA 2Neuro-ophthalmology Division, Department of Neurology, Hospital of the University of Pennsylvania, Philadelphia, PA, USA INTRODUCTION light without distortion (Maurice, 1970). The tear–air interface and cornea contribute more to the focusing Visual processing poses an enormous computational of light than the lens does; unlike the lens, however, the challenge for the brain, which has evolved highly focusing power of the cornea is fixed. The ciliary mus- organized and efficient neural systems to meet these cles dynamically adjust the shape of the lens in order demands. In primates, approximately 55% of the cortex to focus light optimally from varying distances upon is specialized for visual processing (compared to 3% for the retina (accommodation). The total amount of light auditory processing and 11% for somatosensory pro- reaching the retina is controlled by regulation of the cessing) (Felleman and Van Essen, 1991). Over the past pupil aperture. Ultimately, the visual image becomes several decades there has been an explosion in scientific projected upside-down and backwards on to the retina understanding of these complex pathways and net- (Fishman, 1973). works. Detailed knowledge of the anatomy of the visual The majority of the blood supply to structures of the system, in combination with skilled examination, allows eye arrives via the ophthalmic artery, which is the first precise localization of neuropathological processes. -

Rapid Evolution of the Visual System: a Cellular Assay of the Retina and Dorsal Lateral Geniculate Nucleus of the Spanish Wildcat and the Domestic Cat
The Journal of Neuroscience, January 1993, 13(l): 208-229 Rapid Evolution of the Visual System: A Cellular Assay of the Retina and Dorsal Lateral Geniculate Nucleus of the Spanish Wildcat and the Domestic Cat Robert W. Williams,’ Carmen Cavada,2 and Fernando Reinoso-Suhrez* ‘Department of Anatomy and Neurobiology, College of Medicine, University of Tennessee, Memphis, Tennessee 38163 and *Departamento de Morfologia, Facultad de Medicina, Universidad Aut6noma de Madrid, 28029 Madrid, Spain The large Spanish wildcat, Fe/is silvestris tartessia, has re- and important topic, it has been difficult to study the process tained features of the Pleistocene ancestor of the modern of brain evolution in any detail. Our approach has been to domestic cat, F. catus. To gauge the direction and magnitude identify a pair of closely related living species,one from a highly of short-term evolutionary change in this lineage, we have conservative branch that has retained near identity with the compared the retina, the optic nerve, and the dorsal lateral ancestral species,and the other from a derived branch that has geniculate nucleus (LGN) of Spanish wildcats and their do- undergone rapid evolutionary change. The recent recognition mestic relatives. Retinas of the two species have the same that evolution and speciationcan occur in short bursts separated area. However, densities of cone photoreceptors are higher by long interludes of stasisprovides a sound theoretical basis in wildcat-over 100% higher in the area centralis-where- for a search for such pairs (Schindewolf, 1950; Eldredge and as rod densities are as high, or higher, in the domestic lin- Gould, 1972; Stanley, 1979; Gould and Eldredge, 1986). -

Physiology of the Retina
PHYSIOLOGY OF THE RETINA András M. Komáromy Michigan State University [email protected] 12th Biannual William Magrane Basic Science Course in Veterinary and Comparative Ophthalmology PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS • NON-NEURONAL RETINAL CELLS • RETINAL BLOOD FLOW Retina ©Webvision Retina Retinal pigment epithelium (RPE) Photoreceptor segments Outer limiting membrane (OLM) Outer nuclear layer (ONL) Outer plexiform layer (OPL) Inner nuclear layer (INL) Inner plexiform layer (IPL) Ganglion cell layer Nerve fiber layer Inner limiting membrane (ILM) ©Webvision Inherited Retinal Degenerations • Retinitis pigmentosa (RP) – Approx. 1 in 3,500 people affected • Age-related macular degeneration (AMD) – 15 Mio people affected in U.S. www.nei.nih.gov Mutations Causing Retinal Disease http://www.sph.uth.tmc.edu/Retnet/ Retina Optical Coherence Tomography (OCT) Histology Monkey (Macaca fascicularis) fovea Ultrahigh-resolution OCT Drexler & Fujimoto 2008 9 Adaptive Optics Roorda & Williams 1999 6 Types of Retinal Neurons • Photoreceptor cells (rods, cones) • Horizontal cells • Bipolar cells • Amacrine cells • Interplexiform cells • Ganglion cells Signal Transmission 1st order SPECIES DIFFERENCES!! Photoreceptors Horizontal cells 2nd order Bipolar cells Amacrine cells 3rd order Retinal ganglion cells Visual Pathway lgn, lateral geniculate nucleus Changes in Membrane Potential Net positive charge out Net positive charge in PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS -

The Horizontal Raphe of the Human Retina and Its Watershed Zones
vision Review The Horizontal Raphe of the Human Retina and its Watershed Zones Christian Albrecht May * and Paul Rutkowski Department of Anatomy, Medical Faculty Carl Gustav Carus, TU Dresden, 74, 01307 Dresden, Germany; [email protected] * Correspondence: [email protected] Received: 24 September 2019; Accepted: 6 November 2019; Published: 8 November 2019 Abstract: The horizontal raphe (HR) as a demarcation line dividing the retina and choroid into separate vascular hemispheres is well established, but its development has never been discussed in the context of new findings of the last decades. Although factors for axon guidance are established (e.g., slit-robo pathway, ephrin-protein-receptor pathway) they do not explain HR formation. Early morphological organization, too, fails to establish a HR. The development of the HR is most likely induced by the long posterior ciliary arteries which form a horizontal line prior to retinal organization. The maintenance might then be supported by several biochemical factors. The circulation separate superior and inferior vascular hemispheres communicates across the HR only through their anastomosing capillary beds resulting in watershed zones on either side of the HR. Visual field changes along the HR could clearly be demonstrated in vascular occlusive diseases affecting the optic nerve head, the retina or the choroid. The watershed zone of the HR is ideally protective for central visual acuity in vascular occlusive diseases but can lead to distinct pathological features. Keywords: anatomy; choroid; development; human; retina; vasculature 1. Introduction The horizontal raphe (HR) was first described in the early 1800s as a horizontal demarcation line that extends from the macula to the temporal Ora dividing the temporal retinal nerve fiber layer into a superior and inferior half [1]. -
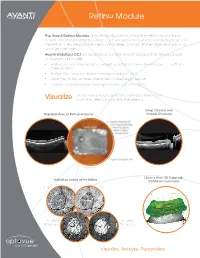
Retina Module Information
Retina Module The Avanti Retina Module gives Retina Specialists new information on structures outside the traditional 6mm x 6mm cube, provides assessment of individual layers of the retina, offers views of the vitreous and deep choroid, and enables evaluation of change over time. Avanti Widefield OCT incorporates a number of technologies that deliver clinical and practical benefits. • High-speed scanning produces exquisitely detailed 12mm x 9mm images in less than three seconds • Motion Correction Technology minimizes motion artifact • Real-time tracking enables assessment of disease progression • A range of reports allow personalized views of retinal anatomy Retinal anatomy beyond the standard 6mm scan Visualize from the deep choroid into the vitreous Deep Choroid and Widefield Views of Retinal Anatomy Vitreous Structures 12mm x 9mm 3D Cube with Individual Layers of the Retina 100 Million Data Points ILM IPL RPE Offset RPE Offset 60 microns 90 microns Visualize. Analyze. Personalize Retina Module Analyze Personalize Retinal structures with comprehensive reports Your view of the retina to optimize treatment planning and patient outcomes Assess Track Change Retinal Assessment Multiple views of the retina in a single, easy-to-read report In foveal thickness and macular volume The Avanti Retina Module offers a range of scans to provide extensive information about retinal health. • 3D Widefield scan displays 9mm x 12mm views of the retina with minimal artifact. • Crossline, grid, raster and radial scans offer unique perspectives on retinal structures. • En face viewing displays individual layers of the retina for assessment of micro-changes. • 3mm scan depth reveals structures from the deep choroid to the vitreous. -

Posterior Vitreous Detachment (PVD) Is
RETINA HEALTH SERIES | Facts from the ASRS The Foundation American Society of Retina Specialists Committed to improving the quality of life of all people with retinal disease. Posterior Vitreous Detachment (PVD) is SYMPTOMS IN DETAIL a natural change that occurs during adulthood, when the vitreous gel that fills the eye separates from the retina, Mild floaters in the vision are the light-sensing nerve layer at the back of the eye. normal, but a sudden increase in floaters is often the first Symptoms of a PVD include: symptom of PVD. Floaters are • Floaters (mobile blurry shadows that obscure the vision) most bothersome when near the • Flashes (streaks of light, usually at the side of the vision) center of vision and less annoying These symptoms usually become less intense over several weeks. when they settle to the side of the vision. They may appear like Most patients experience PVD after age 60, once in each eye, and the cobwebs, dust, or a swarm of condition is usually non-sight-threatening but occasionally affects vision more insects — or in the shape of a permanently in the event of complication, such as retinal detachment circle or oval, called a Weiss ring. or epiretinal membrane. During PVD, floaters are often accompanied by flashes, which Causes: Over time, the vitreous gel that fills the eye becomes liquid and are most noticeable in dark condenses (shrinks) due to age and normal wear and tear. Eventually it surroundings. Most patients cannot fill the whole volume of the eye’s vitreous cavity (which remains the experience floaters and flashes same size during adulthood) and so the gel separates from the retina, located during the first few weeks of a PVD, at the very back of the eye cavity. -

Pupillometry: Psychology, Physiology, and Function
journal of cognition Mathôt, S. 2018 Pupillometry: Psychology, Physiology, and Function. Journal of Cognition, 1(1): 16, pp. 1–23, DOI: https://doi.org/10.5334/joc.18 REVIEW ARTICLE Pupillometry: Psychology, Physiology, and Function Sebastiaan Mathôt Rijksuniversiteit Groningen, NL [email protected] Pupils respond to three distinct kinds of stimuli: they constrict in response to brightness (the pupil light response), constrict in response to near fixation (the pupil near response), and dilate in response to increases in arousal and mental effort, either triggered by an external stimulus or spontaneously. In this review, I describe these three pupil responses, how they are related to high-level cognition, and the neural pathways that control them. I also discuss the functional relevance of pupil responses, that is, how pupil responses help us to better see the world. Although pupil responses likely serve many functions, not all of which are fully under- stood, one important function is to optimize vision either for acuity (small pupils see sharper) and depth of field (small pupils see sharply at a wider range of distances), or for sensitivity (large pupils are better able to detect faint stimuli); that is, pupils change their size to optimize vision for a particular situation. In many ways, pupil responses are similar to other eye move- ments, such as saccades and smooth pursuit: like these other eye movements, pupil responses have properties of both reflexive and voluntary action, and are part of active visual exploration. Keywords: pupillometry; pupil light response; pupil near response; psychosensory pupil response; orienting response; eye movements Seeing is an activity. -
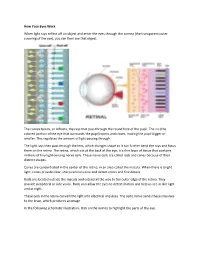
How Your Eyes Work When Light Rays Reflect Off an Object and Enter The
How Your Eyes Work When light rays reflect off an object and enter the eyes through the cornea (the transparent outer covering of the eye), you can then see that object. The cornea bends, or refracts, the rays that pass through the round hole of the pupil. The iris (the colored portion of the eye that surrounds the pupil) opens and closes, making the pupil bigger or smaller. This regulates the amount of light passing through. The light rays then pass through the lens, which changes shape so it can further bend the rays and focus them on the retina. The retina, which sits at the back of the eye, is a thin layer of tissue that contains millions of tiny light-sensing nerve cells. These nerve cells are called rods and cones because of their distinct shapes. Cones are concentrated in the center of the retina, in an area called the macula. When there is bright light, cones provide clear, sharp central vision and detect colors and fine details. Rods are located outside the macula and extend all the way to the outer edge of the retina. They provide peripheral or side vision. Rods also allow the eyes to detect motion and help us see in dim light and at night. These cells in the retina convert the light into electrical impulses. The optic nerve sends these impulses to the brain, which produces an image. In the following schematic illustration, click on the names to highlight the parts of the eye. . -

Introduction to Retina
Human Eye ANAT 3045 Visual Neuroscience BIOS 3001 Advanced Visual Neuroscience Introduction to the Retina Professor Tom Salt UCL Institute of Ophthalmology [email protected] Institute of Ophthalmology Section through the human eye with schematic enlargement of the retina Human Retina Neurotransmitters in Retina Schematic vertical section Light Microscopic vertical section Organisation of neurotransmitters according to cell type in mammalian retina LIGHT Photoreceptors Rod / Cone Distribution Rods always form a hexagonal packing around the cones and separate the cones from each other Four photoreceptor types in human retina: Short-wavelength (S, blue) cones, middle/medium wavelength cones (M, green), long wavelength cones (L, red) and rods. Rods peak in density in a ring approximately 5mm (18 degrees) from the center of the fovea (human/rhesus Dim / Dark (scotopic) conditions – rod function dominates monkey). Rods are present in greater numbers than cones from 2 mm from the Light / ”normal” (photopic) conditions – cone function dominates fovea to the far periphery. 1 Photoreceptor responses Photoreceptor responses - rods An intracellular recording from a single cone stimulated with different amounts of light (cone from the turtle retina). Each trace represents the response to a brief flash that was varied in intensity. At the highest light levels, the response amplitude saturates (at about -65 mV). Photovoltages recorded in monkey rods with suction electrodes. The hyperpolarizing response is characteristic of vertebrate photoreceptors. The granularity of response to dim light stimuli is evident. (After Schnapf and Baylor, 1987.) Photoreceptor responses - cones Photoreceptor responses - summary • In the dark (i.e. absence of light, a.k.a. OFF), photoreceptors have a depolarised cell membrane potential and are releasing their neurotransmitter (L- glutamate) continuously.