The Role of Circular Folds in Mixing Intensification in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Digestive System
Connective tissue The Digestive System Part 1 Structure of digestive system Functions Basic Structure of the Alimentary Canal Wall Tube is made up of four layers: 1. Mucosa 2. Submucosa 3. Muscularis externa 4. Serosa (Peritoneum) or Adventitia Mucosa The innermost wall of the alimentary tube. Consists of: • Epithelium - usually simple columnar epithelium with goblet cells; may be stratified squamous if protection is needed (e.g. esophagus) • Lamina propria – loose connective tissue • Muscularis mucosae – takes part in the formation of folds Submucosa Made up of loose connective tissue. Contains submucosal (Meissner’s) nervous plexus and blood vessels, sometimes glands. Muscularis externa Usually two layers of smooth muscle: • inner circular layer • outer longitudinal layer. • Myenteric (Auerbach’s) nervous plexus in between • Responsible for peristalsis (controlled by the nerve plexus) Outer membrane • A serous membrane/peritoneum consisting of the mesothelium (simple squamous epithelium), and a small amount of underlying loose connective tissue. • Or adventitia consisting only of connective tissue is found where the wall of the tube is directly attached or fixed to adjoining structures (i.e., body wall and certain organs). Enteric nervous system The Alimentary Canal Pharynx Common respiratory and digestive pathway (both air and swallowed food and drinks pass through). • Stratified squamous non-keratinized epithelium • Lamina propria contains many elastic fibers • No muscularis mucosae • No submucosa • Striated muscle in the muscularis externa Esophagus Fixed muscular tube that delivers food and liquid from the pharynx to the stomach. Esophagus Epithelium - stratified squamous Mucosal and submucosal glands of the esophagus secrete mucus to lubricate and protect the luminal wall. Esophageal glands proper lie in the submucosa. -

Vestibule Lingual Frenulum Tongue Hyoid Bone Trachea (A) Soft Palate
Mouth (oral cavity) Parotid gland Tongue Sublingual gland Salivary Submandibular glands gland Esophagus Pharynx Stomach Pancreas (Spleen) Liver Gallbladder Transverse colon Duodenum Descending colon Small Jejunum Ascending colon intestine Ileum Large Cecum intestine Sigmoid colon Rectum Appendix Anus Anal canal © 2018 Pearson Education, Inc. 1 Nasopharynx Hard palate Soft palate Oral cavity Uvula Lips (labia) Palatine tonsil Vestibule Lingual tonsil Oropharynx Lingual frenulum Epiglottis Tongue Laryngopharynx Hyoid bone Esophagus Trachea (a) © 2018 Pearson Education, Inc. 2 Upper lip Gingivae Hard palate (gums) Soft palate Uvula Palatine tonsil Oropharynx Tongue (b) © 2018 Pearson Education, Inc. 3 Nasopharynx Hard palate Soft palate Oral cavity Uvula Lips (labia) Palatine tonsil Vestibule Lingual tonsil Oropharynx Lingual frenulum Epiglottis Tongue Laryngopharynx Hyoid bone Esophagus Trachea (a) © 2018 Pearson Education, Inc. 4 Visceral peritoneum Intrinsic nerve plexuses • Myenteric nerve plexus • Submucosal nerve plexus Submucosal glands Mucosa • Surface epithelium • Lamina propria • Muscle layer Submucosa Muscularis externa • Longitudinal muscle layer • Circular muscle layer Serosa (visceral peritoneum) Nerve Gland in Lumen Artery mucosa Mesentery Vein Duct oF gland Lymphoid tissue outside alimentary canal © 2018 Pearson Education, Inc. 5 Diaphragm Falciform ligament Lesser Liver omentum Spleen Pancreas Gallbladder Stomach Duodenum Visceral peritoneum Transverse colon Greater omentum Mesenteries Parietal peritoneum Small intestine Peritoneal cavity Uterus Large intestine Cecum Rectum Anus Urinary bladder (a) (b) © 2018 Pearson Education, Inc. 6 Cardia Fundus Esophagus Muscularis Serosa externa • Longitudinal layer • Circular layer • Oblique layer Body Lesser Rugae curvature of Pylorus mucosa Greater curvature Duodenum Pyloric Pyloric sphincter antrum (a) (valve) © 2018 Pearson Education, Inc. 7 Fundus Body Rugae of mucosa Pyloric Pyloric (b) sphincter antrum © 2018 Pearson Education, Inc. -

The Small and Large Intestines∗
OpenStax-CNX module: m46512 1 The Small and Large Intestines∗ OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0y Abstract By the end of this section, you will be able to: • Compare and contrast the location and gross anatomy of the small and large intestines • Identify three main adaptations of the small intestine wall that increase its absorptive capacity • Describe the mechanical and chemical digestion of chyme upon its release into the small intestine • List three features unique to the wall of the large intestine and identify their contributions to its function • Identify the benecial roles of the bacterial ora in digestive system functioning • Trace the pathway of food waste from its point of entry into the large intestine through its exit from the body as feces The word intestine is derived from a Latin root meaning internal, and indeed, the two organs together nearly ll the interior of the abdominal cavity. In addition, called the small and large bowel, or colloquially the guts, they constitute the greatest mass and length of the alimentary canal and, with the exception of ingestion, perform all digestive system functions. 1 The Small Intestine Chyme released from the stomach enters the small intestine, which is the primary digestive organ in the body. Not only is this where most digestion occurs, it is also where practically all absorption occurs. The longest part of the alimentary canal, the small intestine is about 3.05 meters (10 feet) long in a living person (but about twice as long in a cadaver due to the loss of muscle tone). -

Aandp2ch25lecture.Pdf
Chapter 25 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • Most nutrients we eat cannot be used in existing form – Must be broken down into smaller components before body can make use of them • Digestive system—acts as a disassembly line – To break down nutrients into forms that can be used by the body – To absorb them so they can be distributed to the tissues • Gastroenterology—the study of the digestive tract and the diagnosis and treatment of its disorders 25-2 General Anatomy and Digestive Processes • Expected Learning Outcomes – List the functions and major physiological processes of the digestive system. – Distinguish between mechanical and chemical digestion. – Describe the basic chemical process underlying all chemical digestion, and name the major substrates and products of this process. 25-3 General Anatomy and Digestive Processes (Continued) – List the regions of the digestive tract and the accessory organs of the digestive system. – Identify the layers of the digestive tract and describe its relationship to the peritoneum. – Describe the general neural and chemical controls over digestive function. 25-4 Digestive Function • Digestive system—organ system that processes food, extracts nutrients, and eliminates residue • Five stages of digestion – Ingestion: selective intake of food – Digestion: mechanical and chemical breakdown of food into a form usable by -

Digestive System
Digestive system Dr. Anna L. Kiss Department of Anatomy, Histology and Embryology Semmelweis University Budapest 2019 The gastrointestinal tract (GI tract): digestion and excretion Upper gastrointestinal tract The upper GI tract consists of the mouth, pharynx, esophagus, and stomach. The lower GI tract. small intestine, which has three parts: -duodenum -jejunum -ileum large intestine, which has three parts: -cecum (the vermiform appendix is attached to the cecum). -colon (ascending colon, transverse colon, descending colon and sigmoid flexure) -rectum Primitive Gut Tube Coeliac trunk Superior mesenteric artery Inferior mesenteric artery Vitelline duct Umbilical loop Umbilical artery Final Position of Parts of Gut Tube Abdominal esophagus Thoracic esophagus Liver Stomach Gall bladder & bile duct Duodenum Pancreas 2.) Transverse colon Jejunum & ileum 1.) Ascending colon 3.) Descending colon Cecum Appendix 4.) Sigmoid colon Final Position of Parts of Gut Tube Stomach: left hypochondric region (intraperitoneal) Duodenum: right side (partly retroperitoneal) Jejunum, ileum: umbilical + iliac region (intraperitoneal) Appendix: right side (Mc Burney point) (intraperitoneal) Ascending colon: right iliac region Transverse colon: middle position (intraperitoneal) Descending colon: left iliac region Sigmoid colon: sacral and pelvic region (intraperitoneal) highly acidic environment due to gastric acid production The stomach lies between the esophagus and the duodenum It is on the left side of the abdominal cavity. Stomach fundus cardia rugae!! lesser curvature body pylorus greater curvature Diaphragm Fundus pyloric antrum Corpus superior part body (duodenum) Greater curvature descending part (duodenum) ascending part Jejunum horizontal part Histology of the gut Mucosa: • epithelium: simple columnar (goblet cells) • propria (lymphoreticular connective tissue): glands (Lieberkhün crypts) • muscularis mucosae (2 layered smooth muscle) Submucosa: loose connective tissue (submucosus plexus; glands, lymphatic follicles) External muscle layer (t. -

Large Intestine
Large Intestine The large intestine is the terminal part of the gastrointestinal tract. The primary digestive function of this organ is to finish absorption, produce some vitamins, form feces, resorb water and eliminate feces from the body. The large intestine runs from the cecum, where it attches to the ileum, to the anus. It borders the small intestine on three sides. Despite its being around half as long as the small intestine – 4.9 feet versus 10 feet (1.5 – 3 meters) – it is called the large intestine because it is more than twice the diameter of the small intestine, 2.5 inches versus one inch (6 cm versus 2.5 cm). The large intestine is tethered to the posterior abdominal wall by the mesocolon, a double layer of peritoneal membrane. The large intestine is subdivided into four main regions: the cecum, the colon, the rectum, and the anus. The ileocecal valve, located at the opening between the ileum in the small intestine and the large intestine, controls the flow of chyme from the small to the large intestine. Large Intestine Anatomical Structures Like the small intestine, the mucosa of the large intestine has intestinal glands that contain both absorptive and goblet cells. However, there are several notable differences between the walls of the large and small intestines. For example, other than the anal canal, the mucosa of the colon is simple columnar epithelium. In addition, the wall of the large intestine has no circular folds, no villi, and essentially no enzyme- secreting cells. This is because most nutrients are already absorbed before chyme enters the large intestine. -

GIT: Esophagus-Stomach- Small Intestine
GIT: Esophagus-Stomach- Small Intestine MUDr. Azzat Al-Redouan Overview of the anatomical development Primitive gut- 4 Weeks Organs Derivatives Foregut → Esophagus ↘ Duodenum ↗ Midgut → Small Intestine ↘ Large Intestine ↗ Hindgut Organ Differentiation and Proliferation ➢ Endodermal inner epithelium → endothelial layer of mucosa, ducts and glands. ➢ Splanchnopleuric mesenchyme → lamina propria and muscularis, submucosa, external muscles and connective tissue. ➢ Splanchnopleuric coelomic epithelium → outer peritonial epithelium. ➢ Local population of angiogenic mesenchyme → blood vessels and lymphatics. ➢ Neural crest → enteric and autonomic nervous system. The sequential genetic expression basics Hedgehog (Hh) Ligands Mutational Endodermal epithelium Endothelial cells Knockout Shh Ihh Dhh • Esophageal atrasia Patch1 Receptors • Gut malformation Patch 2 • Defective muscularis propria • Enteric neurone anomalies • Imperforate anus Transcription Promote GIT Factor Gli3 differentiation Esophagus “Oesophagus” 25cm Muscular Tube C6 STARTS Th1 Connects from Pharynx ➢At the level of the inferior border Superior Mediastinum of the cricoid cartilage Th4 Inferior Mediastinum Connects to Stomach Th10 ➢At the gastric cardiac orifice. ENDS Th11 Inferior Thyroid a. Esophageal branches Esophageal branches Esophageal branches anastomoses Thoracic Aorta Thoracic Esophageal branches Ascending branches chain Vascular Inferior phrenic a. Left gastric a. Inferior thyroid v. Bronchial vv. vv. Plexus Plexus Azygous venous network esophagus - Submucous Intercostal vv. Esophageal Peri *A site of the porto-caval anastomoses Oesophageal Varcies Liver disease → ↑Portal resistance ↓ Porto-systemic shunting *A site of the porto-caval anastomoses (short gastric coronary vv ↔ esophageal vv.) Longitudinal continuous submucosal lymphatic system Cervical esophagus → Deep cervical nn. l. ↘ ↑ Paratrachial nn. l. ↗ Thoracic esophagus → posterior mediastinal nn. l. Abdominal esophagus → left gastric nn. l. * Some may pass directly → Thoracic duct ANS: Sympathetic trunk + Vagus n. -
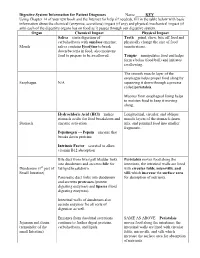
Digestive System Information for Patient Diagnoses
Digestive System Information for Patient Diagnoses Name _____KEY________________ Using Chapter 14 of your text book and the Internet for help (if needed), fill in the table below with basic information about the chemical (enzymes, secretions) impact (if any) and physical/mechanical impact (if any) each of the digestive organs has on food as it passes through our digestive system. Organ Chemical Impact Physical Impact Saliva – starts digestion of Teeth – grind, chew, bite off food and carbohydrates with amylase enzyme; physically change the size of food Mouth saliva contains lysozyme to break (mastication). down bacteria in food, also moistens food to prepare to be swallowed. Tongue – manipulates food and helps form a bolus (food ball) and initiates swallowing. The smooth muscle layer of the esophagus helps propel food along by Esophagus N/A squeezing it down through a process called peristalsis. Mucous from esophageal lining helps to moisten food to keep it moving along. Hydrochloric Acid (HCl) – makes Longitudinal, circular, and oblique stomach acidic for food breakdown and muscle layers of the stomach churn, Stomach enzyme activation mix, and pummel food into smaller fragments. Pepsinogen → Pepsin – enzyme that breaks down proteins Intrinsic Factor – secreted to allow vitamin B12 absorption Bile duct from liver/gall bladder links Peristalsis moves food along the into duodenum and secretes bile for intestines; the intestinal walls are lined Duodenum (1st part of fat/lipid breakdown. with circular folds, microvilli, and Small Intestine) villi which increase the surface area Pancreatic duct links into duodenum for absorption of nutrients. and secretes proteases (protein digesting enzymes) and lipases (lipid digesting enzymes). -

Part 1: Overview of the Digestive System Digestive System: 2 Parts
23 The Digestive System 6 slides Digestive System: 2 parts 1. alimentary canal aka gastrointestinal (GI) tract or gut continuous, muscular digestive Part 1: tube about 20" long Overview of the open at both ends (mouth and anus) so food in the tube is Digestive System technically outside the body functions: digest (break food down into smaller fragments) and absorb nutrients through lining into blood includes: mouth, pharynx, esophagus, stomach, small intestine, and large intestine Digestive System: 2 parts 1 2. accessory organs includes: teeth, tongue, gallbladder, and digestive glands (salivary glands, liver, pancreas) 2 digestive gland function: produce secretions that contribute to the breakdown of foodstuffs A1 3 A2 A3 4 5 6 7 2 Name this structure. 1 MATA: Which of the following NEVER touch food? A esophagus B liver C pancreas D colon E gallbladder 23 The Digestive System 6 slides 3 Name this structure. 4 Name this structure. Digestive Processes Digestive Processes 1. ingestion - taking food into the digestive tract 3. mechanical digestion - physically prepares food 2. propulsion - moves food through alimentary canal for chemical digestion (increases surface area) swallowing - voluntary ex. chewing, stomach churning, segmentation peristalsis - involuntary; alternating waves of 4. chemical digestion - breaks food down into their contraction and relaxation that squeezes food chemical building blocks along GI tract accomplished by enzymes starts in mouth and ends in small intestine 5. absorption - passage of nutrients through alimentary canal wall into blood or lymph most occurs in small intestine 6. defecation - elimination of indigestible substances (fecal material) through anus Click for animation 1 2 4 5 3 Mechanical digestion Chemical digestion *increases surface area exposed *changes molecular form 6 23 The Digestive System 6 slides WINNER...ingestion, propulsion, defecation NONWINNER...mechanical & chemical breakdown, Feeling lucky?...choose ODD OR EVEN absorption 5 Increases surface area of food. -

Gastrointestinal Tract 4: Anatomy and Role of the Jejunum and Ileum
Copyright EMAP Publishing 2019 This article is not for distribution except for journal club use Clinical Practice Keywords Villi/Microvilli/Absorption/ Segmentation/Vitamin B complex Systems of life This article has been GI tract double-blind peer reviewed In this article... ● Role of the jejunum and ileum in chemical digestion and absorption of nutrients ● Nutrient absorption from the small intestine to the bloodstream via the villi ● Processes of segmentation and peristalsis Gastrointestinal tract 4: anatomy and role of the jejunum and ileum Key points Authors Yamni Nigam is professor in biomedical science; John Knight is associate The small intestine professor in biomedical science; Nikki Williams is associate professor in respiratory comprises the physiology; all at the College of Human and Health Sciences, Swansea University. duodenum, jejunum and ileum Abstract After its passage through the duodenum, where most chemical digestion takes place, chyme passes through the jejunum and ileum. Their main role is to ensure The jejunum and that the various molecules resulting from chemical digestion pass through the gut ileum finish chemical wall into the blood or lymph. This process of nutrient absorption is helped by the digestion and presence of folds and projections that hugely increase the surface area of the gut absorb most of wall, and regular contractions of the rings of smooth muscle that move intestinal the nutrients contents back and forth. This article, the fourth in a six-part series exploring the gastrointestinal tract, describes the anatomy and functions of the jejunum and ileum. Folds and projections in the Citation Nigam Y et al (2019) Gastrointestinal tract 4: anatomy and role of the small intestine’s wall jejunum and ileum. -

Lips* Cheeks* Hard / Soft Palate Uvula
DIGESTIVE ANATOMY REVIEW GUIDE GROSS ANATOMY Oral Cavity* (Figure / Cadaver* ) Teeth (Figure) Lips* Incisors / Canines / Premolars / Molars Cheeks* Crown Hard / Soft palate Enamel Uvula Gingiva Tongue Root Palatoglossal arches Cementum Palatopharyngeal arches Periodontal ligaments Lingual frenulum Dentin Pulp cavity Esophagus (Model) Root canal Cardiac sphincter Salivary Glands* (Figure / Cadaver*) Stomach* (Figure / Model / Cadaver*) Parotid glands* Greater / Lesser curvatures* Parotid duct Greater / Lesser omentum Submandibular glands* Cardia* / Fundus* / Body* / Pylorus* Submandibular duct Rugae Sublingual glands Pyloric sphincter* Liver* (Figure / Model / Cadaver*) Small Intestine* (Figure / Model / Cadaver*) Right / Left / Caudate / Quadrate lobes* Duodenum* / Jejunum / Ileum* Falciform ligament* Mesentery* Common hepatic duct Ileocecal valve* Common bile duct* Circular folds Hepatopancreatic ampulla Large Intestine* (Figure / Model / Cadaver*) Duodenal papilla Cecum* Gallbladder* (Model / Figure / Cadaver*) Appendix Cystic duct* Ascending / Transverse colon* Descending / Sigmoid colon* Pancreas* (Figure / Model / Cadaver*) Teniae coli* Pancreatic duct Haustra* Mesocolon Rectum External / Internal anal sphincter BI 335 – Advanced Human Anatomy and Physiology Western Oregon University DIGESTIVE ANATOMY REVIEW GUIDE HISTOLOGY Alimentary Canal (Histological image / slide) Small Intestine (Histological image / slide) Mucosa Mucosa Epithelium Simple columnar epithelium Lamina propria -

ANATOMY Question
NAME:- EMMA-OJOBAH. I. DIVINE-FAVOUR COURSE CODE:- ANA 204 MAT NO:- 18/MHS03/003 DEPT:- ANATOMY Question:- What Are Intestines? The muscular tube that stretches from the lower end of the stomach to the anus is called intestine. It is also known as the bowel or bowels. Further, the intestine is divided into the small intestine and large intestine. Although both these intestines are interconnected, they can be differentiated in many ways. In this article, we discuss about the difference between the small intestine and large intestine. The Small Intestine? The small intestine is made up of three segments, which form a passage from your stomach (the opening between your stomach and small intestine is called the pylorus) to your large intestine: THE STRUCTURE The coiled tube of the small intestine is subdivided into three regions. From proximal (at the stomach) to distal, these are the duodenum, jejunum, and ileum 1. The Duodenum:- is short section is the part of the small intestine that takes in semi- digested food from your stomach through the pylorus, and continues the digestion process. The duodenum also uses bile from your gallbladder, liver, and pancreas to help digest food. 2. The Jejunum:- The middle section of the small intestine carries food through rapidly, with wave-like muscle contractions, towards the ileum. 3. The Ileum:- This last section is the longest part of your small intestine. The ileum is where most of the nutrients from your food are absorbed before emptying into the large intestine. By the time food reaches your small intestine, it has already been broken up and mashed into liquid by your stomach.