2012 American College of Rheumatology Guidelines for Management of Gout. Part 2: Therapy and Antiinflammatory Prophylaxis Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Juvenile Spondyloarthropathies: Inflammation in Disguise
PP.qxd:06/15-2 Ped Perspectives 7/25/08 10:49 AM Page 2 APEDIATRIC Volume 17, Number 2 2008 Juvenile Spondyloarthropathieserspective Inflammation in DisguiseP by Evren Akin, M.D. The spondyloarthropathies are a group of inflammatory conditions that involve the spine (sacroiliitis and spondylitis), joints (asymmetric peripheral Case Study arthropathy) and tendons (enthesopathy). The clinical subsets of spondyloarthropathies constitute a wide spectrum, including: • Ankylosing spondylitis What does spondyloarthropathy • Psoriatic arthritis look like in a child? • Reactive arthritis • Inflammatory bowel disease associated with arthritis A 12-year-old boy is actively involved in sports. • Undifferentiated sacroiliitis When his right toe starts to hurt, overuse injury is Depending on the subtype, extra-articular manifestations might involve the eyes, thought to be the cause. The right toe eventually skin, lungs, gastrointestinal tract and heart. The most commonly accepted swells up, and he is referred to a rheumatologist to classification criteria for spondyloarthropathies are from the European evaluate for possible gout. Over the next few Spondyloarthropathy Study Group (ESSG). See Table 1. weeks, his right knee begins hurting as well. At the rheumatologist’s office, arthritis of the right second The juvenile spondyloarthropathies — which are the focus of this article — toe and the right knee is noted. Family history is might be defined as any spondyloarthropathy subtype that is diagnosed before remarkable for back stiffness in the father, which is age 17. It should be noted, however, that adult and juvenile spondyloar- reported as “due to sports participation.” thropathies exist on a continuum. In other words, many children diagnosed with a type of juvenile spondyloarthropathy will eventually fulfill criteria for Antinuclear antibody (ANA) and rheumatoid factor adult spondyloarthropathy. -
Uncontrolled Gout Fact Sheet
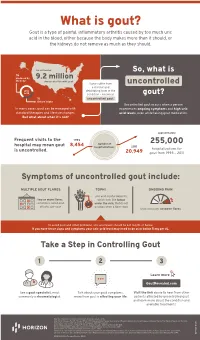
What is gout? Gout is a type of painful, inflammatory arthritis caused by too much uric acid in the blood, either because the body makes more than it should, or the kidneys do not remove as much as they should. An estimated So, what is ⅔ produced by 9.2 million the body Americans live with gout Some suffer from uncontrolled a chronic and uric debilitating form of the acid condition – known as gout? ⅓ uncontrolled gout. dietary intake Uncontrolled gout occurs when a person In many cases, gout can be managed with experiences ongoing symptoms and high uric standard therapies and lifestyle changes. acid levels, even while taking gout medication. But what about when it’s not? approximately Frequent visits to the 1993 number of 255,000 hospital may mean gout 8,454 hospitalizations 2011 is uncontrolled. hospitalizations for 20,949 gout from 1993 – 2011 Symptoms of uncontrolled gout include: MULTIPLE GOUT FLARES TOPHI ONGOING PAIN uric acid crystal deposits, two or more flares, which look like lumps sometimes called gout under the skin, that do not attacks, per year go away when a flare stops that continues between flares To avoid gout and other problems, uric acid levels should be 6.0 mg/dL or below. If you have these signs and symptoms your uric acid level may need to be at or below 5 mg per dL. Take a Step in Controlling Gout 1 2 3 Learn more GoutRevealed.com See a gout specialist, most Talk about your gout symptoms, Visit the link above to hear from other commonly a rheumatologist. -
Arthritis (Overview)
ARTHRITIS Having arthritis can significantly affect your comfort & ability to walk and move with confidence. This is because it affects your joints, which are responsible for keeping you steady and moving efficiently. Your symptoms and causes will depend on the type of arthritis that you have. At Masterton Foot Clinic, our podiatrists work closely with patients with four types of arthritis. OSTEOARTHRITIS Osteoarthritis is the wear and tear arthritis that develops slowly over time as the cartilage that covers your bone ends wears down. The cause is largely from natural use over many years, though injuries, alignment issues within the joint and other diseases may result in it developing at a faster rate. We work with patients that want to feel more comfortable on their feet, despite having arthritis in their hip, knee, ankle and foot joints. RHEUMATOID ARTHRITIS Rheumatoid arthritis is an autoimmune disease that affects the joints. It occurs when your body’s immune system attacks the joints and causes damage, inflammation and pain. If the effects of rheumatoid arthritis remain uncontrolled, it can cause permanent changes in the appearance of the joints, especially at the feet and hands. We work with patients to help them manage the discomfort associated with rheumatoid arthritis, offloading prominent and painful areas that have developed due to changes in the joints. GOUT Gout is an inflammatory arthritis that results from a high concentration of uric acid in the blood. It is associated with a high intake of purine-containing foods like red meats, shellfish and red wine, hence it was previously referred to as the rich man’s disease. -

Gout and Monoarthritis
Gout and Monoarthritis Acute monoarthritis has numerous causes, but most commonly is related to crystals (gout and pseudogout), trauma and infection. Early diagnosis is critical in order to identify and treat septic arthritis, which can lead to rapid joint destruction. Joint aspiration is the gold standard method of diagnosis. For many reasons, managing gout, both acutely and as a chronic disease, is challenging. Registrars need to develop a systematic approach to assessing monoarthritis, and be familiar with the management of gout and other crystal arthropathies. TEACHING AND • Aetiology of acute monoarthritis LEARNING AREAS • Risk factors for gout and septic arthritis • Clinical features and stages of gout • Investigation of monoarthritis (bloods, imaging, synovial fluid analysis) • Joint aspiration techniques • Interpretation of synovial fluid analysis • Management of hyperuricaemia and gout (acute and chronic), including indications and targets for urate-lowering therapy • Adverse effects of medications for gout, including Steven-Johnson syndrome • Indications and pathway for referral PRE- SESSION • Read the AAFP article - Diagnosing Acute Monoarthritis in Adults: A Practical Approach for the Family ACTIVITIES Physician TEACHING TIPS • Monoarthritis may be the first symptom of an inflammatory polyarthritis AND TRAPS • Consider gonococcal infection in younger patients with monoarthritis • Fever may be absent in patients with septic arthritis, and present in gout • Fleeting monoarthritis suggests gonococcal arthritis or rheumatic fever -

19120 Arthritis Aus Gout Booklet
Taking control of your Gout A practical guide to treatments, services and lifestyle choices How can this booklet help you This booklet is designed for people who have gout. It will help you understand your • make healthy choices for your condition so that you can better general health and wellbeing manage your symptoms and continue • find support and additional to lead an active and healthy life. information to cope with the This booklet offers information and impact of gout. practical advice to help you: The information inside is based • understand what gout is and on the latest research and what it means for you recommendations, and has been reviewed by Australian experts in the • understand how medicines can field of arthritis to make sure it is help treat gout attacks and current and relevant to your needs. prevent future attacks So go ahead — take control of • work with your healthcare team your gout! to manage the disease in the short and long term © Copyright Arthritis Australia 2014 Supported by: AstraZeneca Pty Limited ABN 54 009 682 311 Alma Road, North Ryde NSW 2113 2 Taking control of your Gout Contents Understanding gout 4 Treating gout 10 Diet and lifestyle 16 Who can help? 21 Working with your GP 22 Seeing a rheumatologist 23 Other health professionals 24 Seeking support 26 Glossary of terms 28 Useful resources 29 Medical and consumer consultants Tanya deKroo, Information Resources Coordinator, Arthritis Australia Wendy Favorito, Arthritis Australia Consumer Representative and Board Member Assoc Prof Neil McGill, Rheumatologist Assoc Prof Julian McNeil, Rheumatologist and Chair of Australian Rheumatology Association’s Therapeutics Committee Assoc Prof Peter Youssef, Rheumatologist and Chair of Arthritis Australia’s Scientific Advisory Committee Arthritis Australia 3 Understanding gout What is gout? the main reason for more than Gout is an extremely painful form nine out of ten people with gout). -

21362 Arthritis Australia a to Z List
ARTHRITISINFORMATION SHEET Here is the A to Z of arthritis! A D Goodpasture’s syndrome Achilles tendonitis Degenerative joint disease Gout Achondroplasia Dermatomyositis Granulomatous arteritis Acromegalic arthropathy Diabetic finger sclerosis Adhesive capsulitis Diffuse idiopathic skeletal H Adult onset Still’s disease hyperostosis (DISH) Hemarthrosis Ankylosing spondylitis Discitis Hemochromatosis Anserine bursitis Discoid lupus erythematosus Henoch-Schonlein purpura Avascular necrosis Drug-induced lupus Hepatitis B surface antigen disease Duchenne’s muscular dystrophy Hip dysplasia B Dupuytren’s contracture Hurler syndrome Behcet’s syndrome Hypermobility syndrome Bicipital tendonitis E Hypersensitivity vasculitis Blount’s disease Ehlers-Danlos syndrome Hypertrophic osteoarthropathy Brucellar spondylitis Enteropathic arthritis Bursitis Epicondylitis I Erosive inflammatory osteoarthritis Immune complex disease C Exercise-induced compartment Impingement syndrome Calcaneal bursitis syndrome Calcium pyrophosphate dehydrate J (CPPD) F Jaccoud’s arthropathy Crystal deposition disease Fabry’s disease Juvenile ankylosing spondylitis Caplan’s syndrome Familial Mediterranean fever Juvenile dermatomyositis Carpal tunnel syndrome Farber’s lipogranulomatosis Juvenile rheumatoid arthritis Chondrocalcinosis Felty’s syndrome Chondromalacia patellae Fibromyalgia K Chronic synovitis Fifth’s disease Kawasaki disease Chronic recurrent multifocal Flat feet Kienbock’s disease osteomyelitis Foreign body synovitis Churg-Strauss syndrome Freiberg’s disease -
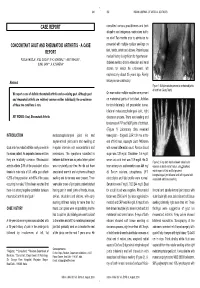
CONCOMITANT GOUT and RHEUMATOID ARTHRITIS - a CASE Presented with Multiple Nodular Swellings on REPORT Feet, Hands, Wrists and Elbows
349 350 INDIAN JOURNAL OF MEDICAL SCIENCES CASE REPORT consulted various practitioners and took allopathic and indigenous medications but to no relief. Two months prior to admission he CONCOMITANT GOUT AND RHEUMATOID ARTHRITIS - A CASE presented with multiple nodular swellings on REPORT feet, hands, wrists and elbows. Patient’s past medical history is significant for hypertension, POOJA KHOSLA*, ATUL GOGIA**, P. K. AGARWAL***, AMIT PAHUJA**, SUNIL JAIN***, K. K. SAXENA# diabetes mellitus, chronic ethanolism and renal stones for which he underwent left nephrectomy about 25 years ago. Family Abstract history is non-contributory. Figure 1: Multiple nodules present on metatarsal joints of both feet (Gouty Tophi) We report a case of definite rheumatoid arthritis and co-existing gout. Although gout On examination multiple nodules were present and rheumatoid arthritis are relatively common entities individually, the co-existence on metatarsal joints of both feet, Achilles of these two conditions is rare. tendon bilaterally, left prepatellar bursa, bilateral metacarpophalangeal joint, right KEY WORDS: Gout, Rheumatoid Arthritis olecranon process. There was swelling and tenderness of PIP and MCP joints of both feet. (Figure 1) Laboratory data revealed INTRODUCTION metatarsophalangeal joint. He had hemoglobin - 8.9gm/dl, ESR 124 mm at the asymmetrical joint pains and swelling at end of first hour, leucocyte count 7400/cmm Gout and rheumatoid arthritis rarely co-exist in irregular intervals with exacerbations and with normal differential count. Random blood the same patient. As separate disease entities remissions. The symptoms subsided in sugar was 139 mg/dl, Creatinine- 1.6 mg/dl; they are relatively common. Rheumatoid between but there was no period when patient serum uric acid level was 10.9 mg/dl. -

Psoriasis, Psoriatic Arthritis and Secondary Gout – 3 Case Reports
https://doi.org/10.5272/jimab.2018242.1972 Journal of IMAB Journal of IMAB - Annual Proceeding (Scientific Papers). 2018 Apr-Jun;24(2) ISSN: 1312-773X https://www.journal-imab-bg.org Case report PSORIASIS, PSORIATIC ARTHRITIS AND SECONDARY GOUT – 3 CASE REPORTS Mina I. Ivanova, Rositsa Karalilova, Zguro Batalov. Departement of Rheumatology, Faculty of Medicine, UMHAT Kaspela, Medical University - Plovdiv, Bulgaria. ABSTRACT: cells in the skin (Langerhans cells) migrate to the regional Background: One of the most common complica- lymph nodes, where interacts with T-lymphocytes. This tions in psoriasis is the development of secondary gout that provokes the immune response leading to the activation often remains undiagnosed for many years. In some cases, of T cells and release of cytokines. The local effects of the clinical symptoms of gout precede the manifestation cytokines lead to a cell-mediated immune response. In pso- of cutaneous psoriasis, leading to progression of the dis- riasis skin lesions are results of hyperplasia of the epider- ease and early onset of complications. According to the mis, which leads to enhanced cell reproduction, world data, there is a strong correlation between psoriasis hyperproliferation and at the same time shortening vulgaris and psoriatic arthritis on the one hand and gout keratinocytes’ life. This leads to increased production of on the other ranging from 3 to 40%. In Bulgaria, there are uric acid, as it enhances the exchange of nucleic acids. no studies observing the frequency of secondary gout in Psoriatic arthritis (PsA) occurs in 0.05 to 0.25% from psoriatic patients. the general population. -

The Patient's Guide to Treating and Managing Gout
Raising the Voice of Patients THE PATIENT’S GUIDE TO TREATING AND MANAGING GOUT First Edition ©2018 GLOBAL HEALTHY LIVING FOUNDATION, INC. Table of Contents 2 PART ONE Introduction 3 PART TWO Patient Charter 6 PART THREE Treatment Guidelines 7 PART FOUR Gout Overview 11 PART FIVE Monitoring 12 PART SIX Treatments Treatments for acute gout flares Colchicine (Colcrys®, Mitigare®) NSAIDS Corticosteroids Corticotropin or Adrenocorticotrophic Hormone (Acthar®, H.P. Acthar Gel®) Xanthine Oxidase Inhibitors Allopurinol (Aloprim®, Lopurim®, Zyloprim®) Febuxostat (Uloric®) Uricosoric Medications Probenecid (Benemid®, Probalan®) Lesinurad (Zurampic®, Duzallo®) Uricase Medications Pegloticase (Krystexxa®) Off-Label Gout Treatments: Anakinra and Canakinumab Surgery 29 PART SEVEN Self Management 35 PART EIGHT Affording Your Treatments 37 PART NINE Make Your Voice Heard 41 PART TEN Conclusion 42 ABOUT THE EDITORS 43 GLOSSARY 44 WORKS REFERENCED THE PATIENT’S GUIDE TO TREATING AND MANAGING GOUT | 1 PART ONE Welcome Welcome to the first edition of CreakyJoints’ patient guide for living with and managing gout. This original guide offers comprehensive, easy-to-understand information on gout and its treatment: what causes gout, how it can affect your body in different ways and progress, how it’s treated and the steps you can take on your own to prevent flares and manage your symptoms. CreakyJoints is a non-profit patient advocacy organization that empowers patients like you to raise your voice with key decision-makers you’ll encounter while living with gout. This guide is the first of its kind, and it has been developed by leading experts, including doctors who specialize in gout management, other healthcare providers and patients like you. -

Understanding Gout and Your Kidneys
UNDERSTANDINGUnderstanding GOUT ANDYour YOUR KIDNEYS Hemodialysis Access Options UNDERSTANDING GOUT AND YOUR KIDNEYS WHAT IS GOUT? Gout happens if a substance called uric acid gets too high in your blood. Gout is a common form of arthritis. Everyone has small amounts of uric It can cause pain, swelling, and acid in their blood. It comes from redness in your joints. Gout usually two places—the normal breakdown begins in the big toe, but can strike of your body’s cells and certain other joints such as the ankle, knee, foods. At normal levels, uric acid wrist, fingers, or elbow. Usually does not cause any damage. But if only one joint is affected, but some the levels get too high, it can form people may have gout in more than sharp crystals that build up in your one joint, especially if it is not found body’s joints and cause pain. The and treated. medical term for high blood urate levels is hyperuricemia. DID YOU KNOW? • About 8.3 million U.S. adults HOW DOES URIC ACID (3.9 percent) are living with CAUSE GOUT? gout today and up to 10% of people worldwide may have Whenever you eat or drink gout. Gout is the most common something, your body pulls out the form of arthritis. good stuff like vitamins, and gets rid of the waste. One of those • Gout puts you at risk for kidney waste products is uric acid. It is disease. Up to 20% percent of made when your body breaks down people with gout have mild to purines, which is found in certain moderate kidney disease. -

Psoriatic Arthritisdth 1306 123..136
Dermatologic Therapy, Vol. 23, 2010, 123–136 © 2010 Wiley Periodicals, Inc. Printed in the United States · All rights reserved DERMATOLOGIC THERAPY ISSN 1396-0296 Psoriatic arthritisdth_1306 123..136 Uwe Wollina*, Leonore Unger†, Birgit Heinig‡ and Thomas Kittner§ *Department of Dermatology and Allergology, †Department of Rheumatology, ‡Centre for Physical Therapy and Rehabilitation, and §Department of Radiology, Hospital Dresden-Friedrichstadt, Academic Teaching Hospital of the Technical University of Dresden, Dresden, Germany ABSTRACT: Psoriatic arthritis (PSA) is an entity of inflammatory joint disease associated with psoria- sis. PSA belongs to the heterogeneous group of seronegative spondylarthropathies. Both peripheral joints and axial skeleton can be affected in a characteristic pattern. In addition to that, enthesitis and dactylitis are important extracutaneous manifestations. Uveitis anterior is temporarily seen in about one quarter of PSA patients. There is a closer relationship of nail and joint disease. This review provides data on drug and physical treatment options. In particular DMARDS and inhibitors of tumor necrosis factor a are established therapies with importance for quality of life and long term outcome. New drugs are tested in various trials. KEYWORDS: biologics, dactylitis, DMARDS, enthesitis, psoriatic arthritis, treatment, uveitis History, definition, and classification Table 1. Classification criteria for psoriatic arthri- of psoriatic arthritis tis (CASPAR) 1. Criterion – inflammatory disease of joints, spine or The possible relationship between psoriasis and tendons/enthesis (2 points) inflammatory joint disease was recognized first by and French medicine since early 19th century (1,2). In 2. At least one of the following criteria (1 point each) 1973, Moll and Wright defined psoriatic arthritis 1. Psoriasis (skin, scalp) – now (PSA) as: inflammatory arthritis (peripheral) and/ 2. -

Hemarthrosis
Case Report DOI: 10.5455/amaj.2016.02.012 Hemarthrosis: Concurrent acute presentation of pyrophosphate dehydrate and uric acid crystals in an elderly patient with a history of rheumatoid ar- thritis diagnosed with septic arthritis Feredun Azari, BS-MD Rauf Shahbazov, MD, PhD* Concomitant septic arthritis in the presence of crystalline disease is a rare presen- tation of acute hemarthrosis and knee pain. Literature review showed that co-occur- Department of Surgery, rence of these entities is an infrequent phenomenon but it needs to be acknowledged University of Virginia, that these studies are few in number and were done on small patient population. This Charlottesville, Virginia, USA case challenges the notion that presence of crystals in the synovial fluid rules out sep- tic arthritis even in the setting of low synovial WBC count. Additionally, the presence Correspondence: of pseudogout in patients suffering from gout is a rare entity as well. These findings Rauf Shahbazov MD, PhD, Organ Trans- in literature are described in case reports dispersed over the past three decades. We plantation Unit, University of Virginia present a case where concurrent treatment of gout, pseudogout, and septic arthritis Medical Center, Charlottesville, Virginia, in a patient who presented with acute hemarhtrosis. USA email: [email protected] Keywords: gout, pseudogout, rheumatoid arthritis, infection Phone: 434-872-1373 Introduction tions [1]. Vigilance is needed to have a low ge related crystal induced arthropathies threshold for ruling out septic arthritis in Aare a common phenomenon presenting this patient population. This is usually done to the primary care office or the emergency via synovial fluid analysis but as demon- department [1,2].