Psoriatic Arthritisdth 1306 123..136
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Juvenile Spondyloarthropathies: Inflammation in Disguise
PP.qxd:06/15-2 Ped Perspectives 7/25/08 10:49 AM Page 2 APEDIATRIC Volume 17, Number 2 2008 Juvenile Spondyloarthropathieserspective Inflammation in DisguiseP by Evren Akin, M.D. The spondyloarthropathies are a group of inflammatory conditions that involve the spine (sacroiliitis and spondylitis), joints (asymmetric peripheral Case Study arthropathy) and tendons (enthesopathy). The clinical subsets of spondyloarthropathies constitute a wide spectrum, including: • Ankylosing spondylitis What does spondyloarthropathy • Psoriatic arthritis look like in a child? • Reactive arthritis • Inflammatory bowel disease associated with arthritis A 12-year-old boy is actively involved in sports. • Undifferentiated sacroiliitis When his right toe starts to hurt, overuse injury is Depending on the subtype, extra-articular manifestations might involve the eyes, thought to be the cause. The right toe eventually skin, lungs, gastrointestinal tract and heart. The most commonly accepted swells up, and he is referred to a rheumatologist to classification criteria for spondyloarthropathies are from the European evaluate for possible gout. Over the next few Spondyloarthropathy Study Group (ESSG). See Table 1. weeks, his right knee begins hurting as well. At the rheumatologist’s office, arthritis of the right second The juvenile spondyloarthropathies — which are the focus of this article — toe and the right knee is noted. Family history is might be defined as any spondyloarthropathy subtype that is diagnosed before remarkable for back stiffness in the father, which is age 17. It should be noted, however, that adult and juvenile spondyloar- reported as “due to sports participation.” thropathies exist on a continuum. In other words, many children diagnosed with a type of juvenile spondyloarthropathy will eventually fulfill criteria for Antinuclear antibody (ANA) and rheumatoid factor adult spondyloarthropathy. -
Uncontrolled Gout Fact Sheet
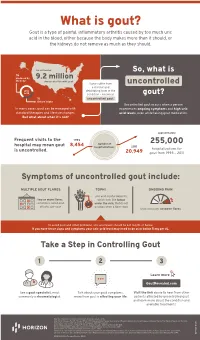
What is gout? Gout is a type of painful, inflammatory arthritis caused by too much uric acid in the blood, either because the body makes more than it should, or the kidneys do not remove as much as they should. An estimated So, what is ⅔ produced by 9.2 million the body Americans live with gout Some suffer from uncontrolled a chronic and uric debilitating form of the acid condition – known as gout? ⅓ uncontrolled gout. dietary intake Uncontrolled gout occurs when a person In many cases, gout can be managed with experiences ongoing symptoms and high uric standard therapies and lifestyle changes. acid levels, even while taking gout medication. But what about when it’s not? approximately Frequent visits to the 1993 number of 255,000 hospital may mean gout 8,454 hospitalizations 2011 is uncontrolled. hospitalizations for 20,949 gout from 1993 – 2011 Symptoms of uncontrolled gout include: MULTIPLE GOUT FLARES TOPHI ONGOING PAIN uric acid crystal deposits, two or more flares, which look like lumps sometimes called gout under the skin, that do not attacks, per year go away when a flare stops that continues between flares To avoid gout and other problems, uric acid levels should be 6.0 mg/dL or below. If you have these signs and symptoms your uric acid level may need to be at or below 5 mg per dL. Take a Step in Controlling Gout 1 2 3 Learn more GoutRevealed.com See a gout specialist, most Talk about your gout symptoms, Visit the link above to hear from other commonly a rheumatologist. -
Arthritis (Overview)
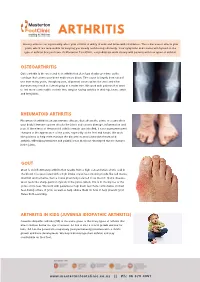
ARTHRITIS Having arthritis can significantly affect your comfort & ability to walk and move with confidence. This is because it affects your joints, which are responsible for keeping you steady and moving efficiently. Your symptoms and causes will depend on the type of arthritis that you have. At Masterton Foot Clinic, our podiatrists work closely with patients with four types of arthritis. OSTEOARTHRITIS Osteoarthritis is the wear and tear arthritis that develops slowly over time as the cartilage that covers your bone ends wears down. The cause is largely from natural use over many years, though injuries, alignment issues within the joint and other diseases may result in it developing at a faster rate. We work with patients that want to feel more comfortable on their feet, despite having arthritis in their hip, knee, ankle and foot joints. RHEUMATOID ARTHRITIS Rheumatoid arthritis is an autoimmune disease that affects the joints. It occurs when your body’s immune system attacks the joints and causes damage, inflammation and pain. If the effects of rheumatoid arthritis remain uncontrolled, it can cause permanent changes in the appearance of the joints, especially at the feet and hands. We work with patients to help them manage the discomfort associated with rheumatoid arthritis, offloading prominent and painful areas that have developed due to changes in the joints. GOUT Gout is an inflammatory arthritis that results from a high concentration of uric acid in the blood. It is associated with a high intake of purine-containing foods like red meats, shellfish and red wine, hence it was previously referred to as the rich man’s disease. -

Adult Still's Disease
44 y/o male who reports severe knee pain with daily fevers and rash. High ESR, CRP add negative RF and ANA on labs. Edward Gillis, DO ? Adult Still’s Disease Frontal view of the hands shows severe radiocarpal and intercarpal joint space narrowing without significant bony productive changes. Joint space narrowing also present at the CMC, MCP and PIP joint spaces. Diffuse osteopenia is also evident. Spot views of the hands after Tc99m-MDP injection correlate with radiographs, showing significantly increased radiotracer uptake in the wrists, CMC, PIP, and to a lesser extent, the DIP joints bilaterally. Tc99m-MDP bone scan shows increased uptake in the right greater than left shoulders, as well as bilaterally symmetric increased radiotracer uptake in the elbows, hands, knees, ankles, and first MTP joints. Note the absence of radiotracer uptake in the hips. Patient had bilateral total hip arthroplasties. Not clearly evident are bilateral shoulder hemiarthroplasties. The increased periprosthetic uptake could signify prosthesis loosening. Adult Stills Disease Imaging Features • Radiographs – Distinctive pattern of diffuse radiocarpal, intercarpal, and carpometacarpal joint space narrowing without productive bony changes. Osseous ankylosis in the wrists common late in the disease. – Joint space narrowing is uniform – May see bony erosions. • Tc99m-MDP Bone Scan – Bilaterally symmetric increased uptake in the small and large joints of the axial and appendicular skeleton. Adult Still’s Disease General Features • Rare systemic inflammatory disease of unknown etiology • 75% have onset between 16 and 35 years • No gender, race, or ethnic predominance • Considered adult continuum of JIA • Triad of high spiking daily fevers with a skin rash and polyarthralgia • Prodromal sore throat is common • Negative RF and ANA Adult Still’s Disease General Features • Most commonly involved joint is the knee • Wrist involved in 74% of cases • In the hands, interphalangeal joints are more commonly affected than the MCP joints. -

Nasal Septal Perforation: a Novel Clinical Manifestation of Systemic Juvenile Idiopathic Arthritis/Adult Onset Still’S Disease
Case Report Nasal Septal Perforation: A Novel Clinical Manifestation of Systemic Juvenile Idiopathic Arthritis/Adult Onset Still’s Disease TADEJ AVCIN,ˇ EARL D. SILVERMAN, VITO FORTE, and RAYFEL SCHNEIDER ABSTRACT. Nasal septal perforation has been well recognized in patients with various rheumatic diseases. To our knowledge, this condition has not been reported in children with systemic juvenile idiopathic arthri- tis (SJIA) or patients with adult onset Still’s disease (AOSD). We describe 3 patients with persistent SJIA/AOSD who developed nasal septal perforation during the course of their disease. As illustrat- ed by these cases, nasal septal perforation may develop as a rare complication of SJIA/AOSD and can be considered as part of the clinical spectrum of the disease. In one case the nasal septal perfo- ration was associated with vasculitis. (J Rheumatol 2005;32:2429–31) Key Indexing Terms: SYSTEMIC JUVENILE IDIOPATHIC ARTHRITIS ADULT ONSET STILL’S DISEASE NASAL SEPTAL PERFORATION Perforation of the nasal septum has been well recognized in tory manifestations to SJIA and may occur at all ages9. We patients with various rheumatic diseases, including describe 3 patients with persistent SJIA/AOSD who devel- Wegener’s granulomatosis, systemic lupus erythematosus oped nasal septal perforation during the course of their dis- (SLE), and sarcoidosis1,2. Nasal involvement is one of the ease (Table 1). major features of Wegener’s granulomatosis and may lead to massive destruction of the septal cartilage and saddle-nose CASE REPORTS deformity3. Nasal septal perforation is also a recognized Case 1. A 4.5-year-old girl first presented in February 1998 with an 8 week complication of mucosal involvement in SLE and occurs in history of spiking fever, evanescent rash, and polyarthritis. -

Nail Psoriasis and Psoriatic Arthritis for the Dermatologist
Liu R, Candela BM, English JC. Nail Psoriasis and Psoriatic Arthritis for the Dermatologist. J Dermatol & Skin Sci. 2020;2(1):17-21 Mini-Review Article Open Access Nail Psoriasis and Psoriatic Arthritis for the Dermatologist Rebecca Liu1, Braden M. Candela2, Joseph C English III2* 1University of Pittsburgh School of Medicine 2Department of Dermatology, University of Pittsburgh, Pittsburgh, PA Article Info Abstract Article Notes Psoriatic arthritis (PsA) may affect up to a third of patients with psoriasis. Received: February 16, 2020 It is characterized by diverse clinical phenotypes and as such, is often Accepted: March 17, 2020 underdiagnosed, leading to disease progression and poor outcomes. Nail *Correspondence: psoriasis (NP) has been identified as a risk factor for PsA, given the anatomical Joseph C. English, MD, PhD, University of Pittsburgh Medical connection between the extensor tendon and nail matrix. Therefore, it Center (UPMC), Department of Dermatology, 9000 Brooktree is important for dermatologists to screen patients exhibiting symptoms Rd, Suite 200, Wexford, PA 15090; Email: [email protected]. of NP for joint manifestations. On physical exam, physicians should be evaluating for concurrent skin and nail involvement, enthesitis, dactylitis, ©2020 English JC. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License. and spondyloarthropathy. Imaging modalities, including radiographs and ultrasound, may also be helpful in diagnosis of both nail and joint pathology. Keywords: Physicians should refer to Rheumatology when appropriate. Numerous Nail psoriasis systemic therapies are effective at addressing both NP and PsA including Psoriatic arthritis DMARDs, biologics, and small molecule inhibitors. These treatments ultimately Psoriasis can inhibit the progression of inflammatory disease and control symptoms, Treatment Management thereby improving quality of life for patients. -

Gout and Monoarthritis
Gout and Monoarthritis Acute monoarthritis has numerous causes, but most commonly is related to crystals (gout and pseudogout), trauma and infection. Early diagnosis is critical in order to identify and treat septic arthritis, which can lead to rapid joint destruction. Joint aspiration is the gold standard method of diagnosis. For many reasons, managing gout, both acutely and as a chronic disease, is challenging. Registrars need to develop a systematic approach to assessing monoarthritis, and be familiar with the management of gout and other crystal arthropathies. TEACHING AND • Aetiology of acute monoarthritis LEARNING AREAS • Risk factors for gout and septic arthritis • Clinical features and stages of gout • Investigation of monoarthritis (bloods, imaging, synovial fluid analysis) • Joint aspiration techniques • Interpretation of synovial fluid analysis • Management of hyperuricaemia and gout (acute and chronic), including indications and targets for urate-lowering therapy • Adverse effects of medications for gout, including Steven-Johnson syndrome • Indications and pathway for referral PRE- SESSION • Read the AAFP article - Diagnosing Acute Monoarthritis in Adults: A Practical Approach for the Family ACTIVITIES Physician TEACHING TIPS • Monoarthritis may be the first symptom of an inflammatory polyarthritis AND TRAPS • Consider gonococcal infection in younger patients with monoarthritis • Fever may be absent in patients with septic arthritis, and present in gout • Fleeting monoarthritis suggests gonococcal arthritis or rheumatic fever -

Differential Diagnosis of Juvenile Idiopathic Arthritis
pISSN: 2093-940X, eISSN: 2233-4718 Journal of Rheumatic Diseases Vol. 24, No. 3, June, 2017 https://doi.org/10.4078/jrd.2017.24.3.131 Review Article Differential Diagnosis of Juvenile Idiopathic Arthritis Young Dae Kim1, Alan V Job2, Woojin Cho2,3 1Department of Pediatrics, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea, 2Department of Orthopaedic Surgery, Albert Einstein College of Medicine, 3Department of Orthopaedic Surgery, Montefiore Medical Center, New York, USA Juvenile idiopathic arthritis (JIA) is a broad spectrum of disease defined by the presence of arthritis of unknown etiology, lasting more than six weeks duration, and occurring in children less than 16 years of age. JIA encompasses several disease categories, each with distinct clinical manifestations, laboratory findings, genetic backgrounds, and pathogenesis. JIA is classified into sev- en subtypes by the International League of Associations for Rheumatology: systemic, oligoarticular, polyarticular with and with- out rheumatoid factor, enthesitis-related arthritis, psoriatic arthritis, and undifferentiated arthritis. Diagnosis of the precise sub- type is an important requirement for management and research. JIA is a common chronic rheumatic disease in children and is an important cause of acute and chronic disability. Arthritis or arthritis-like symptoms may be present in many other conditions. Therefore, it is important to consider differential diagnoses for JIA that include infections, other connective tissue diseases, and malignancies. Leukemia and septic arthritis are the most important diseases that can be mistaken for JIA. The aim of this review is to provide a summary of the subtypes and differential diagnoses of JIA. (J Rheum Dis 2017;24:131-137) Key Words. -

B27 Positive Diseases Versus B27 Negative Diseases
Ann Rheum Dis: first published as 10.1136/ard.47.5.431 on 1 May 1988. Downloaded from Annals of the Rheumatic Diseases, 1988; 47, 431-439 Viewpoint B27 positive diseases versus B27 negative diseases A LINSSEN AND TE W FELTKAMP From the Netherlands Ophthalmic Research Institute, Amsterdam Key words: ankylosing spondylitis, Reiter's syndrome, reactive arthritis, acute anterior uveitis, HLA-B27. Of all the known associations between HLA and indicated genes other than B27.t"' 12 Nor did any of diseases, the association of B27 with ankylosing the B27 subtypes show a particular association with spondylitis (AS) is the strongest. The B27 antigen is AS. 1 8 present in over 90% of patients with AS as com- Strong evidence for B27 as the major genetic pared with the B27 prevalence of 8% in Caucasians susceptibility factor is found in detailed population in general. A strong but slightly minor association and family studies, in which no stronger association copyright. has also been found between B27 and Reiter's has been observed other than that between B27 and syndrome (RS), reactive arthritis (ReA), and acute AS. A stronger B27 association has been found in anterior uveitis (AAU). These diseases are so Caucasians with spondylitis than in their American strongly interrelated, especially in the presence of black counterparts.'9 B27, that one may speak of'B27 associated diseases'. I In addition to genetic factors, infectious agents Many authors regard psoriatic arthritis, also, as a have been regarded as a likely cause of primary AS. disease associated -

Treatment of Refractory Pityriasis Rubra Pilaris with Novel Phosphodiesterase 4
Letters Discussion | Acrodermatitis continua of Hallopeau, also Additional Contributions: We thank the patient for granting permission to known as acrodermatitis perstans and dermatitis repens, publish this information. is a rare inflammatory pustular dermatosis of the distal fin- 1. Saunier J, Debarbieux S, Jullien D, Garnier L, Dalle S, Thomas L. Acrodermatitis continua of Hallopeau treated successfully with ustekinumab gers and toes. It is considered a variant of pustular psoriasis and acitretin after failure of tumour necrosis factor blockade and anakinra. or, less commonly, its own pustular psoriasis-like indepen- Dermatology. 2015;230(2):97-100. 1 dent entity. Precise pathophysiology and incidence 2. Kiszewski AE, De Villa D, Scheibel I, Ricachnevsky N. An infant with are unknown. Case literature suggests predominance in acrodermatitis continua of Hallopeau: successful treatment with thalidomide women, but the disease affects both sexes and, rarely, and UVB therapy. Pediatr Dermatol. 2009;26(1):105-106. children.2 3. Jo SJ, Park JY, Yoon HS, Youn JI. Case of acrodermatitis continua accompanied by psoriatic arthritis. J Dermatol. 2006;33(11):787-791. Acrodermatitis continua of Hallopeau initially presents 4. Sehgal VN, Verma P, Sharma S, et al. Acrodermatitis continua of Hallopeau: as erythema overlying the distal digits that evolves into evolution of treatment options. Int J Dermatol. 2011;50(10):1195-1211. pustules.2 The nail bed is often involved, with paronychial 5. Lutz V, Lipsker D. Acitretin- and tumor necrosis factor inhibitor-resistant 3 and subungual involvement and atrophic skin changes. acrodermatitis continua of Hallopeau responsive to the interleukin 1 receptor Most patients experience a chronic, relapsing course involv- antagonist anakinra. -

19120 Arthritis Aus Gout Booklet
Taking control of your Gout A practical guide to treatments, services and lifestyle choices How can this booklet help you This booklet is designed for people who have gout. It will help you understand your • make healthy choices for your condition so that you can better general health and wellbeing manage your symptoms and continue • find support and additional to lead an active and healthy life. information to cope with the This booklet offers information and impact of gout. practical advice to help you: The information inside is based • understand what gout is and on the latest research and what it means for you recommendations, and has been reviewed by Australian experts in the • understand how medicines can field of arthritis to make sure it is help treat gout attacks and current and relevant to your needs. prevent future attacks So go ahead — take control of • work with your healthcare team your gout! to manage the disease in the short and long term © Copyright Arthritis Australia 2014 Supported by: AstraZeneca Pty Limited ABN 54 009 682 311 Alma Road, North Ryde NSW 2113 2 Taking control of your Gout Contents Understanding gout 4 Treating gout 10 Diet and lifestyle 16 Who can help? 21 Working with your GP 22 Seeing a rheumatologist 23 Other health professionals 24 Seeking support 26 Glossary of terms 28 Useful resources 29 Medical and consumer consultants Tanya deKroo, Information Resources Coordinator, Arthritis Australia Wendy Favorito, Arthritis Australia Consumer Representative and Board Member Assoc Prof Neil McGill, Rheumatologist Assoc Prof Julian McNeil, Rheumatologist and Chair of Australian Rheumatology Association’s Therapeutics Committee Assoc Prof Peter Youssef, Rheumatologist and Chair of Arthritis Australia’s Scientific Advisory Committee Arthritis Australia 3 Understanding gout What is gout? the main reason for more than Gout is an extremely painful form nine out of ten people with gout). -

21362 Arthritis Australia a to Z List
ARTHRITISINFORMATION SHEET Here is the A to Z of arthritis! A D Goodpasture’s syndrome Achilles tendonitis Degenerative joint disease Gout Achondroplasia Dermatomyositis Granulomatous arteritis Acromegalic arthropathy Diabetic finger sclerosis Adhesive capsulitis Diffuse idiopathic skeletal H Adult onset Still’s disease hyperostosis (DISH) Hemarthrosis Ankylosing spondylitis Discitis Hemochromatosis Anserine bursitis Discoid lupus erythematosus Henoch-Schonlein purpura Avascular necrosis Drug-induced lupus Hepatitis B surface antigen disease Duchenne’s muscular dystrophy Hip dysplasia B Dupuytren’s contracture Hurler syndrome Behcet’s syndrome Hypermobility syndrome Bicipital tendonitis E Hypersensitivity vasculitis Blount’s disease Ehlers-Danlos syndrome Hypertrophic osteoarthropathy Brucellar spondylitis Enteropathic arthritis Bursitis Epicondylitis I Erosive inflammatory osteoarthritis Immune complex disease C Exercise-induced compartment Impingement syndrome Calcaneal bursitis syndrome Calcium pyrophosphate dehydrate J (CPPD) F Jaccoud’s arthropathy Crystal deposition disease Fabry’s disease Juvenile ankylosing spondylitis Caplan’s syndrome Familial Mediterranean fever Juvenile dermatomyositis Carpal tunnel syndrome Farber’s lipogranulomatosis Juvenile rheumatoid arthritis Chondrocalcinosis Felty’s syndrome Chondromalacia patellae Fibromyalgia K Chronic synovitis Fifth’s disease Kawasaki disease Chronic recurrent multifocal Flat feet Kienbock’s disease osteomyelitis Foreign body synovitis Churg-Strauss syndrome Freiberg’s disease