Pachydermoperiostosis (Touraine–Solente–Gole Syndrome): a Case
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Juvenile Spondyloarthropathies: Inflammation in Disguise
PP.qxd:06/15-2 Ped Perspectives 7/25/08 10:49 AM Page 2 APEDIATRIC Volume 17, Number 2 2008 Juvenile Spondyloarthropathieserspective Inflammation in DisguiseP by Evren Akin, M.D. The spondyloarthropathies are a group of inflammatory conditions that involve the spine (sacroiliitis and spondylitis), joints (asymmetric peripheral Case Study arthropathy) and tendons (enthesopathy). The clinical subsets of spondyloarthropathies constitute a wide spectrum, including: • Ankylosing spondylitis What does spondyloarthropathy • Psoriatic arthritis look like in a child? • Reactive arthritis • Inflammatory bowel disease associated with arthritis A 12-year-old boy is actively involved in sports. • Undifferentiated sacroiliitis When his right toe starts to hurt, overuse injury is Depending on the subtype, extra-articular manifestations might involve the eyes, thought to be the cause. The right toe eventually skin, lungs, gastrointestinal tract and heart. The most commonly accepted swells up, and he is referred to a rheumatologist to classification criteria for spondyloarthropathies are from the European evaluate for possible gout. Over the next few Spondyloarthropathy Study Group (ESSG). See Table 1. weeks, his right knee begins hurting as well. At the rheumatologist’s office, arthritis of the right second The juvenile spondyloarthropathies — which are the focus of this article — toe and the right knee is noted. Family history is might be defined as any spondyloarthropathy subtype that is diagnosed before remarkable for back stiffness in the father, which is age 17. It should be noted, however, that adult and juvenile spondyloar- reported as “due to sports participation.” thropathies exist on a continuum. In other words, many children diagnosed with a type of juvenile spondyloarthropathy will eventually fulfill criteria for Antinuclear antibody (ANA) and rheumatoid factor adult spondyloarthropathy. -
Hypertrichosis Terminalis
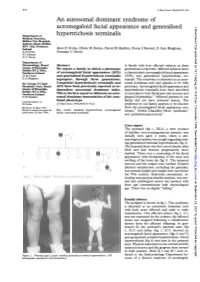
97297 Med Genet 1996;33:972-974 An autosomal dominant syndrome of acromegaloid facial appearance and generalised J Med Genet: first published as 10.1136/jmg.33.11.972 on 1 November 1996. Downloaded from Department of hypertrichosis terminalis Medical Genetics, Belfast City Hospital, Lisburn Road, Belfast BT9 7AB, Northern Ireland Alan D Irvine, Olivia M Dolan, David R Hadden, Fiona J Stewart, E Ann Bingham, A D Irvine Norman C Nevin F J Stewart N C Nevin Department of Dermatology, Royal Abstract A family with four affected subjects in three Group of Hospitals, Belfast BT12 6BA, We report a family in which a phenotype generations is reported. Affected subjects show Northern Ireland of acromegaloid facial appearance (AFA) a characteristic acromegaloid facial appearance 0 M Dolan and generalised hypertrichosis terminalis (AFA) and generalised hypertrichosis ter- E A Bingham segregates through three generations. minalis. The syndrome is inherited as an auto- Sir George E Clark Congenital hypertrichosis terminalis and somal dominant trait and appears to be fully Metabolic Unit, Royal AFA have been previously reported as in- penetrant. Acromegaloid facial appearance and Group of Hospitals, dependent autosomal dominant traits. hypertrichosis terminalis have been described Belfast BT12 6BA, Northern Ireland This is the first report to delineate an auto- in association with thickened oral mucosa and D R Hadden somal dominant transmission of the com- gingival hyperplasia.'"2 Affected patients in our Correspondence to: bined phenotype. family did not have intraoral lesions. The Dr Irvine. (JtMed Genet 1996;33:972-974) syndrome in our family appears to be discrete Received 14 May 1996 from the acromegaloid facial appearance syn- Revised version accepted for Key words: terminal hypertrichosis; acromegaloid drome,3 Gorlin-Chaudhry-Moss syndrome,4 publication 28 June 1996 facies; autosomal dominant. -

Adult Still's Disease
44 y/o male who reports severe knee pain with daily fevers and rash. High ESR, CRP add negative RF and ANA on labs. Edward Gillis, DO ? Adult Still’s Disease Frontal view of the hands shows severe radiocarpal and intercarpal joint space narrowing without significant bony productive changes. Joint space narrowing also present at the CMC, MCP and PIP joint spaces. Diffuse osteopenia is also evident. Spot views of the hands after Tc99m-MDP injection correlate with radiographs, showing significantly increased radiotracer uptake in the wrists, CMC, PIP, and to a lesser extent, the DIP joints bilaterally. Tc99m-MDP bone scan shows increased uptake in the right greater than left shoulders, as well as bilaterally symmetric increased radiotracer uptake in the elbows, hands, knees, ankles, and first MTP joints. Note the absence of radiotracer uptake in the hips. Patient had bilateral total hip arthroplasties. Not clearly evident are bilateral shoulder hemiarthroplasties. The increased periprosthetic uptake could signify prosthesis loosening. Adult Stills Disease Imaging Features • Radiographs – Distinctive pattern of diffuse radiocarpal, intercarpal, and carpometacarpal joint space narrowing without productive bony changes. Osseous ankylosis in the wrists common late in the disease. – Joint space narrowing is uniform – May see bony erosions. • Tc99m-MDP Bone Scan – Bilaterally symmetric increased uptake in the small and large joints of the axial and appendicular skeleton. Adult Still’s Disease General Features • Rare systemic inflammatory disease of unknown etiology • 75% have onset between 16 and 35 years • No gender, race, or ethnic predominance • Considered adult continuum of JIA • Triad of high spiking daily fevers with a skin rash and polyarthralgia • Prodromal sore throat is common • Negative RF and ANA Adult Still’s Disease General Features • Most commonly involved joint is the knee • Wrist involved in 74% of cases • In the hands, interphalangeal joints are more commonly affected than the MCP joints. -

Digital Clubbing
Review Article Digital clubbing Malay Sarkar, D. M. Mahesh1, Irappa Madabhavi2 Department of Pulmonary Medicine, Indira Gandhi Medical College, Shimla, 1Department of Endocrinology, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, 2Post Graduate Student (Medicine), Indira Gandhi Medical College, Shimla, India ABSTRACT Digital clubbing is an ancient and important clinical signs in medicine. Although clubbed fingers are mostly asymptomatic, it often predicts the presence of some dreaded underlying diseases. Its exact pathogenesis is not known, but platelet‑derived growth factor and vascular endothelial growth factor are recently incriminated in its causation. The association of digital clubbing with various disease processes and its clinical implications are discussed in this review. KEY WORDS: Cancer, digital clubbing, hypertrophic osteoarthropathy, megakaryocytes, vascular endothelial growth factor Address for correspondence: Dr. Malay Sarkar, Department of Pulmonary Medicine, Indira Gandhi Medical College, Shimla ‑ 171 001, India. E‑mail: [email protected] INTRODUCTION long bones and occasional painful joint enlargement. It was initially known as hypertrophic pulmonary Digital clubbing is characterized by a focal bulbous osteoarthropathy (HPOA) based on the fact that majority enlargement of the terminal segments of the fingers of cases of HOA are due to malignant thoracic tumors. and/or toes due to proliferation of connective tissue The term “pulmonary” was later abandoned as it was between nail matrix and the distal phalanx. It results in realized that the skeletal syndrome may occur in several increase in both anteroposterior and lateral diameter of non‑pulmonary diseases and even may occur without the nails.[1] Clubbed fingers are also known as watch‑glass any underlying illness. -

Joint Pain Or Joint Disease
ARTHRITIS BY THE NUMBERS Book of Trusted Facts & Figures 2020 TABLE OF CONTENTS Introduction ............................................4 Medical/Cost Burden .................................... 26 What the Numbers Mean – SECTION 1: GENERAL ARTHRITIS FACTS ....5 Craig’s Story: Words of Wisdom What is Arthritis? ...............................5 About Living With Gout & OA ........................ 27 Prevalence ................................................... 5 • Age and Gender ................................................................ 5 SECTION 4: • Change Over Time ............................................................ 7 • Factors to Consider ............................................................ 7 AUTOIMMUNE ARTHRITIS ..................28 Pain and Other Health Burdens ..................... 8 A Related Group of Employment Impact and Medical Cost Burden ... 9 Rheumatoid Diseases .........................28 New Research Contributes to Osteoporosis .....................................9 Understanding Why Someone Develops Autoimmune Disease ..................... 28 Who’s Affected? ........................................... 10 • Genetic and Epigenetic Implications ................................ 29 Prevalence ................................................... 10 • Microbiome Implications ................................................... 29 Health Burdens ............................................. 11 • Stress Implications .............................................................. 29 Economic Burdens ........................................ -

Approach to Polyarthritis for the Primary Care Physician
24 Osteopathic Family Physician (2018) 24 - 31 Osteopathic Family Physician | Volume 10, No. 5 | September / October, 2018 REVIEW ARTICLE Approach to Polyarthritis for the Primary Care Physician Arielle Freilich, DO, PGY2 & Helaine Larsen, DO Good Samaritan Hospital Medical Center, West Islip, New York KEYWORDS: Complaints of joint pain are commonly seen in clinical practice. Primary care physicians are frequently the frst practitioners to work up these complaints. Polyarthritis can be seen in a multitude of diseases. It Polyarthritis can be a challenging diagnostic process. In this article, we review the approach to diagnosing polyarthritis Synovitis joint pain in the primary care setting. Starting with history and physical, we outline the defning characteristics of various causes of arthralgia. We discuss the use of certain laboratory studies including Joint Pain sedimentation rate, antinuclear antibody, and rheumatoid factor. Aspiration of synovial fuid is often required for diagnosis, and we discuss the interpretation of possible results. Primary care physicians can Rheumatic Disease initiate the evaluation of polyarthralgia, and this article outlines a diagnostic approach. Rheumatology INTRODUCTION PATIENT HISTORY Polyarticular joint pain is a common complaint seen Although laboratory studies can shed much light on a possible diagnosis, a in primary care practices. The diferential diagnosis detailed history and physical examination remain crucial in the evaluation is extensive, thus making the diagnostic process of polyarticular symptoms. The vast diferential for polyarticular pain can difcult. A comprehensive history and physical exam be greatly narrowed using a thorough history. can help point towards the more likely etiology of the complaint. The physician must frst ensure that there are no symptoms pointing towards a more serious Emergencies diagnosis, which may require urgent management or During the initial evaluation, the physician must frst exclude any life- referral. -

Nasal Septal Perforation: a Novel Clinical Manifestation of Systemic Juvenile Idiopathic Arthritis/Adult Onset Still’S Disease
Case Report Nasal Septal Perforation: A Novel Clinical Manifestation of Systemic Juvenile Idiopathic Arthritis/Adult Onset Still’s Disease TADEJ AVCIN,ˇ EARL D. SILVERMAN, VITO FORTE, and RAYFEL SCHNEIDER ABSTRACT. Nasal septal perforation has been well recognized in patients with various rheumatic diseases. To our knowledge, this condition has not been reported in children with systemic juvenile idiopathic arthri- tis (SJIA) or patients with adult onset Still’s disease (AOSD). We describe 3 patients with persistent SJIA/AOSD who developed nasal septal perforation during the course of their disease. As illustrat- ed by these cases, nasal septal perforation may develop as a rare complication of SJIA/AOSD and can be considered as part of the clinical spectrum of the disease. In one case the nasal septal perfo- ration was associated with vasculitis. (J Rheumatol 2005;32:2429–31) Key Indexing Terms: SYSTEMIC JUVENILE IDIOPATHIC ARTHRITIS ADULT ONSET STILL’S DISEASE NASAL SEPTAL PERFORATION Perforation of the nasal septum has been well recognized in tory manifestations to SJIA and may occur at all ages9. We patients with various rheumatic diseases, including describe 3 patients with persistent SJIA/AOSD who devel- Wegener’s granulomatosis, systemic lupus erythematosus oped nasal septal perforation during the course of their dis- (SLE), and sarcoidosis1,2. Nasal involvement is one of the ease (Table 1). major features of Wegener’s granulomatosis and may lead to massive destruction of the septal cartilage and saddle-nose CASE REPORTS deformity3. Nasal septal perforation is also a recognized Case 1. A 4.5-year-old girl first presented in February 1998 with an 8 week complication of mucosal involvement in SLE and occurs in history of spiking fever, evanescent rash, and polyarthritis. -

Arthritis and Joint Pain
Fact Sheet News from the IBD Help Center ARTHRITIS AND JOINT PAIN Arthritis, or inflammation (pain with swelling) of the joints, is the most common extraintestinal complication of IBD. It may affect as many as 30% of people with Crohn’s disease or ulcerative colitis. Although arthritis is typically associated with advancing age, in IBD it often strikes younger patients as well. In addition to joint pain, arthritis also causes swelling of the joints and a reduction in flexibility. It is important to point out that people with arthritis may experience arthralgia, but many people with arthralgia may not have arthritis. Types of Arthritis • Peripheral Arthritis. Peripheral arthritis usually affects the large joints of the arms and legs, including the elbows, wrists, knees, and ankles. The discomfort may be “migratory,” moving from one joint to another. If left untreated, the pain may last from a few days to several weeks. Peripheral arthritis tends to be more common among people who have ulcerative colitis or Crohn’s disease of the colon. The level of inflammation in the joints generally mirrors the extent of inflammation in the colon. Although no specific test can make an absolute diagnosis, various diagnostic methods—including analysis of joint fluid, blood tests, and X-rays—are used to rule out other causes of joint pain. Fortunately, IBD-related peripheral arthritis usually does not cause any lasting damage and treatment of the underlying IBD typically results in improvement in the joint discomfort. • Axial Arthritis. Also known as spondylitis or spondyloarthropathy, axial arthritis produces pain and stiffness in the lower spine and sacroiliac joints (at the bottom of the back). -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Nail Psoriasis and Psoriatic Arthritis for the Dermatologist
Liu R, Candela BM, English JC. Nail Psoriasis and Psoriatic Arthritis for the Dermatologist. J Dermatol & Skin Sci. 2020;2(1):17-21 Mini-Review Article Open Access Nail Psoriasis and Psoriatic Arthritis for the Dermatologist Rebecca Liu1, Braden M. Candela2, Joseph C English III2* 1University of Pittsburgh School of Medicine 2Department of Dermatology, University of Pittsburgh, Pittsburgh, PA Article Info Abstract Article Notes Psoriatic arthritis (PsA) may affect up to a third of patients with psoriasis. Received: February 16, 2020 It is characterized by diverse clinical phenotypes and as such, is often Accepted: March 17, 2020 underdiagnosed, leading to disease progression and poor outcomes. Nail *Correspondence: psoriasis (NP) has been identified as a risk factor for PsA, given the anatomical Joseph C. English, MD, PhD, University of Pittsburgh Medical connection between the extensor tendon and nail matrix. Therefore, it Center (UPMC), Department of Dermatology, 9000 Brooktree is important for dermatologists to screen patients exhibiting symptoms Rd, Suite 200, Wexford, PA 15090; Email: [email protected]. of NP for joint manifestations. On physical exam, physicians should be evaluating for concurrent skin and nail involvement, enthesitis, dactylitis, ©2020 English JC. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License. and spondyloarthropathy. Imaging modalities, including radiographs and ultrasound, may also be helpful in diagnosis of both nail and joint pathology. Keywords: Physicians should refer to Rheumatology when appropriate. Numerous Nail psoriasis systemic therapies are effective at addressing both NP and PsA including Psoriatic arthritis DMARDs, biologics, and small molecule inhibitors. These treatments ultimately Psoriasis can inhibit the progression of inflammatory disease and control symptoms, Treatment Management thereby improving quality of life for patients. -

Differential Diagnosis of Juvenile Idiopathic Arthritis
pISSN: 2093-940X, eISSN: 2233-4718 Journal of Rheumatic Diseases Vol. 24, No. 3, June, 2017 https://doi.org/10.4078/jrd.2017.24.3.131 Review Article Differential Diagnosis of Juvenile Idiopathic Arthritis Young Dae Kim1, Alan V Job2, Woojin Cho2,3 1Department of Pediatrics, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea, 2Department of Orthopaedic Surgery, Albert Einstein College of Medicine, 3Department of Orthopaedic Surgery, Montefiore Medical Center, New York, USA Juvenile idiopathic arthritis (JIA) is a broad spectrum of disease defined by the presence of arthritis of unknown etiology, lasting more than six weeks duration, and occurring in children less than 16 years of age. JIA encompasses several disease categories, each with distinct clinical manifestations, laboratory findings, genetic backgrounds, and pathogenesis. JIA is classified into sev- en subtypes by the International League of Associations for Rheumatology: systemic, oligoarticular, polyarticular with and with- out rheumatoid factor, enthesitis-related arthritis, psoriatic arthritis, and undifferentiated arthritis. Diagnosis of the precise sub- type is an important requirement for management and research. JIA is a common chronic rheumatic disease in children and is an important cause of acute and chronic disability. Arthritis or arthritis-like symptoms may be present in many other conditions. Therefore, it is important to consider differential diagnoses for JIA that include infections, other connective tissue diseases, and malignancies. Leukemia and septic arthritis are the most important diseases that can be mistaken for JIA. The aim of this review is to provide a summary of the subtypes and differential diagnoses of JIA. (J Rheum Dis 2017;24:131-137) Key Words. -

B27 Positive Diseases Versus B27 Negative Diseases
Ann Rheum Dis: first published as 10.1136/ard.47.5.431 on 1 May 1988. Downloaded from Annals of the Rheumatic Diseases, 1988; 47, 431-439 Viewpoint B27 positive diseases versus B27 negative diseases A LINSSEN AND TE W FELTKAMP From the Netherlands Ophthalmic Research Institute, Amsterdam Key words: ankylosing spondylitis, Reiter's syndrome, reactive arthritis, acute anterior uveitis, HLA-B27. Of all the known associations between HLA and indicated genes other than B27.t"' 12 Nor did any of diseases, the association of B27 with ankylosing the B27 subtypes show a particular association with spondylitis (AS) is the strongest. The B27 antigen is AS. 1 8 present in over 90% of patients with AS as com- Strong evidence for B27 as the major genetic pared with the B27 prevalence of 8% in Caucasians susceptibility factor is found in detailed population in general. A strong but slightly minor association and family studies, in which no stronger association copyright. has also been found between B27 and Reiter's has been observed other than that between B27 and syndrome (RS), reactive arthritis (ReA), and acute AS. A stronger B27 association has been found in anterior uveitis (AAU). These diseases are so Caucasians with spondylitis than in their American strongly interrelated, especially in the presence of black counterparts.'9 B27, that one may speak of'B27 associated diseases'. I In addition to genetic factors, infectious agents Many authors regard psoriatic arthritis, also, as a have been regarded as a likely cause of primary AS. disease associated