(12) Patent Application Publication (10) Pub. No.: US 2016/0346364 A1 BRUNS Et Al
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
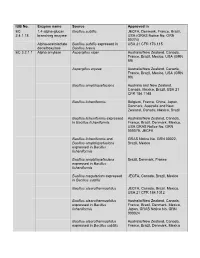
IUB No. Enzyme Name Source Approved in EC 1,4-Alpha-Glucan Bacillus Subtilis JECFA, Denmark, France, Brazil, 2.4.1.18 Branching Enzyme USA (GRAS Notice No
IUB No. Enzyme name Source Approved in EC 1,4-alpha-glucan Bacillus subtilis JECFA, Denmark, France, Brazil, 2.4.1.18 branching enzyme USA (GRAS Notice No. GRN 00274) Alpha-acetolactate Bacillus subtilis expressed in USA 21 CFR 173.115 decarboxylase Bacillus brevis EC 3.2.1.1 Alpha amylase Aspergillus niger Australia/New Zealand, Canada, France, Brazil, Mexico, USA (GRN 89) Aspergillus oryzae Australia/New Zealand, Canada, France, Brazil, Mexico, USA (GRN 90) Bacillus amyloliquefaciens Australia and New Zealand, Canada, Mexico, Brazil, USA 21 CFR 184.1148 Bacillus licheniformis Belgium, France, China, Japan, Denmark, Australia and New Zealand, Canada, Mexico, Brazil Bacillus licheniformis expressed Australia/New Zealand, Canada, in Bacillus licheniformis France, Brazil, Denmark, Mexico, USA GRAS Notice No. GRN 000079, JECFA Bacillus licheniformis and GRAS Notice No. GRN 00022, Bacillus amyloliquefaciens Brazil, Mexico expressed in Bacillus licheniformis Bacillus amyloliquefaciens Brazil, Denmark, France expressed in Bacillus licheniformis Bacillus megaterium expressed JECFA, Canada, Brazil, Mexico in Bacillus subtilis Bacillus stearothermophilus JECFA, Canada, Brazil, Mexico, USA 21 CFR 184.1012 Bacillus stearothermophilus Australia/New Zealand, Canada, expressed in Bacillus France, Brazil, Denmark, Mexico, licheniformis Japan, GRAS Notice No. GRN 000024 Bacillus stearothermophilus Australia/New Zealand, Canada, expressed in Bacillus subtilis France, Brazil, Denmark, Mexico Bacillus stearothermophilus Australia/New Zealand, Canada, (Geobacillus France, Europa, Brazil, Mexico, stearothermophilus) USA (GRN 594) Bacillus subtilis Australia and New Zealand, Canada, Mexico, Brazil, USA 21 CFR 184.1148 Rhizopus delemar Brazil Rhizopus oryzae Brazil, Mexico, USA GRAS Notice No. GRN 000090 Thermoccocales expressed in Brazil, USA (GRN126) Pseudomonas fluorecens Rhizomucor pusillus expressed Brazil, Denmark, France, Mexico, in Aspergillus niger Australia and New Zealand. -

Clinical Study High Complement Factor I Activity in the Plasma of Children with Autism Spectrum Disorders
Hindawi Publishing Corporation Autism Research and Treatment Volume 2012, Article ID 868576, 6 pages doi:10.1155/2012/868576 Clinical Study High Complement Factor I Activity in the Plasma of Children with Autism Spectrum Disorders Naghi Momeni,1 Lars Brudin,2 Fatemeh Behnia,3 Berit Nordstrom,¨ 4 Ali Yosefi-Oudarji,5 Bengt Sivberg,4 Mohammad T. Joghataei,5 and Bengt L. Persson1 1 School of Natural Sciences, Linnaeus University, 39182 Kalmar, Sweden 2 Department of Clinical Physiology, Kalmar County Hospital, 39185 Kalmar, Sweden 3 Department of Occupational Therapy, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran 4 Department of Health Sciences, Autism Research, Faculty of Medicine, Lund University, Box 157, 22100 Lund, Sweden 5 Cellular and Molecular Research Centre, Tehran University of Medical Sciences (TUMS), Tehran, Iran Correspondence should be addressed to Bengt Sivberg, [email protected] Received 17 June 2011; Revised 22 August 2011; Accepted 22 August 2011 Academic Editor: Judy Van de Water Copyright © 2012 Naghi Momeni et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Autism spectrum disorders (ASDs) are neurodevelopmental and behavioural syndromes affecting social orientation, behaviour, and communication that can be classified as developmental disorders. ASD is also associated with immune system abnormality. Im- mune system abnormalities may be caused partly by complement system factor I deficiency. Complement factor I is a serine pro- tease present in human plasma that is involved in the degradation of complement protein C3b, which is a major opsonin of the complement system. -

Mouse Prolyl Endopeptidase / PREP Protein (His Tag)
Mouse Prolyl endopeptidase / PREP Protein (His Tag) Catalog Number: 50288-M07B General Information SDS-PAGE: Gene Name Synonym: AI047692; AI450383; D10Wsu136e; PEP; Pop Protein Construction: A DNA sequence encoding the mature form of mouse PREP (Q9QUR6) (Leu2-Gln710) was expressed, with a polyhistidine tag at the N-terminus. Source: Mouse Expression Host: Baculovirus-Insect Cells QC Testing Purity: > 95 % as determined by SDS-PAGE Endotoxin: Protein Description < 1.0 EU per μg of the protein as determined by the LAL method Prolyl endopeptidase, also known as PREP, belongs to a distinct class of Stability: serine peptidases. It is a large cytosolic enzyme which was first described in the cytosol of rabbit brain as an oligopeptidase. Prolyl endopeptidase ℃ Samples are stable for up to twelve months from date of receipt at -70 degrades the nonapeptide bradykinin at the Pro-Phe bond. It is involved in the maturation and degradation of peptide hormones and neuropeptides His Predicted N terminal: such as alpha-melanocyte-stimulating hormone, luteinizing hormone- Molecular Mass: releasing hormone (LH-RH), thyrotropin-releasing hormone, angiotensin, neurotensin, oxytocin, substance P and vasopressin. Prolyl The recombinant mouse PREP consists of 727 amino acids and predicts a endopeptidase's activity is confined to action on oligopeptides of less than molecular mass of 82.9 KDa. It migrates as an approximately 79-83 KDa 10 kD and it has an absolute requirement for the trans-configuration of the band in SDS-PAGE under reducing conditions. peptide bond preceding proline. It cleaves peptide bonds at the C-terminal side of proline residues. Formulation: References Lyophilized from sterile 20mM Tris, 500mM NaCl, pH 7.4, 10% glycerol, 3mM DTT 1.Oliveira EB, et al. -

Effects of Glycosylation on the Enzymatic Activity and Mechanisms of Proteases
International Journal of Molecular Sciences Review Effects of Glycosylation on the Enzymatic Activity and Mechanisms of Proteases Peter Goettig Structural Biology Group, Faculty of Molecular Biology, University of Salzburg, Billrothstrasse 11, 5020 Salzburg, Austria; [email protected]; Tel.: +43-662-8044-7283; Fax: +43-662-8044-7209 Academic Editor: Cheorl-Ho Kim Received: 30 July 2016; Accepted: 10 November 2016; Published: 25 November 2016 Abstract: Posttranslational modifications are an important feature of most proteases in higher organisms, such as the conversion of inactive zymogens into active proteases. To date, little information is available on the role of glycosylation and functional implications for secreted proteases. Besides a stabilizing effect and protection against proteolysis, several proteases show a significant influence of glycosylation on the catalytic activity. Glycans can alter the substrate recognition, the specificity and binding affinity, as well as the turnover rates. However, there is currently no known general pattern, since glycosylation can have both stimulating and inhibiting effects on activity. Thus, a comparative analysis of individual cases with sufficient enzyme kinetic and structural data is a first approach to describe mechanistic principles that govern the effects of glycosylation on the function of proteases. The understanding of glycan functions becomes highly significant in proteomic and glycomic studies, which demonstrated that cancer-associated proteases, such as kallikrein-related peptidase 3, exhibit strongly altered glycosylation patterns in pathological cases. Such findings can contribute to a variety of future biomedical applications. Keywords: secreted protease; sequon; N-glycosylation; O-glycosylation; core glycan; enzyme kinetics; substrate recognition; flexible loops; Michaelis constant; turnover number 1. -

Progress in the Field of Aspartic Proteinases in Cheese Manufacturing
Progress in the field of aspartic proteinases in cheese manufacturing: structures, functions, catalytic mechanism, inhibition, and engineering Sirma Yegin, Peter Dekker To cite this version: Sirma Yegin, Peter Dekker. Progress in the field of aspartic proteinases in cheese manufacturing: structures, functions, catalytic mechanism, inhibition, and engineering. Dairy Science & Technology, EDP sciences/Springer, 2013, 93 (6), pp.565-594. 10.1007/s13594-013-0137-2. hal-01201447 HAL Id: hal-01201447 https://hal.archives-ouvertes.fr/hal-01201447 Submitted on 17 Sep 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Dairy Sci. & Technol. (2013) 93:565–594 DOI 10.1007/s13594-013-0137-2 REVIEW PAPER Progress in the field of aspartic proteinases in cheese manufacturing: structures, functions, catalytic mechanism, inhibition, and engineering Sirma Yegin & Peter Dekker Received: 25 February 2013 /Revised: 16 May 2013 /Accepted: 21 May 2013 / Published online: 27 June 2013 # INRA and Springer-Verlag France 2013 Abstract Aspartic proteinases are an important class of proteinases which are widely used as milk-coagulating agents in industrial cheese production. They are available from a wide range of sources including mammals, plants, and microorganisms. -

Serine Proteases with Altered Sensitivity to Activity-Modulating
(19) & (11) EP 2 045 321 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.04.2009 Bulletin 2009/15 C12N 9/00 (2006.01) C12N 15/00 (2006.01) C12Q 1/37 (2006.01) (21) Application number: 09150549.5 (22) Date of filing: 26.05.2006 (84) Designated Contracting States: • Haupts, Ulrich AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 51519 Odenthal (DE) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • Coco, Wayne SK TR 50737 Köln (DE) •Tebbe, Jan (30) Priority: 27.05.2005 EP 05104543 50733 Köln (DE) • Votsmeier, Christian (62) Document number(s) of the earlier application(s) in 50259 Pulheim (DE) accordance with Art. 76 EPC: • Scheidig, Andreas 06763303.2 / 1 883 696 50823 Köln (DE) (71) Applicant: Direvo Biotech AG (74) Representative: von Kreisler Selting Werner 50829 Köln (DE) Patentanwälte P.O. Box 10 22 41 (72) Inventors: 50462 Köln (DE) • Koltermann, André 82057 Icking (DE) Remarks: • Kettling, Ulrich This application was filed on 14-01-2009 as a 81477 München (DE) divisional application to the application mentioned under INID code 62. (54) Serine proteases with altered sensitivity to activity-modulating substances (57) The present invention provides variants of ser- screening of the library in the presence of one or several ine proteases of the S1 class with altered sensitivity to activity-modulating substances, selection of variants with one or more activity-modulating substances. A method altered sensitivity to one or several activity-modulating for the generation of such proteases is disclosed, com- substances and isolation of those polynucleotide se- prising the provision of a protease library encoding poly- quences that encode for the selected variants. -

Reducing Immunoreactivity of Gliadins and Coeliac-Toxic Peptides Using Peptidases from L
catalysts Article Reducing Immunoreactivity of Gliadins and Coeliac-Toxic Peptides Using Peptidases from L. acidophilus 5e2 and A. niger Bartosz Brzozowski *, Katarzyna Stasiewicz, Mateusz Ostolski and Marek Adamczak Department of Food Biotechnology, Faculty of Food Sciences, University of Warmia and Mazury in Olsztyn, 10-718 Olsztyn, Poland; [email protected] (K.S.); [email protected] (M.O.); [email protected] (M.A.) * Correspondence: [email protected] Received: 21 July 2020; Accepted: 10 August 2020; Published: 11 August 2020 Abstract: Wheat storage proteins and products of their hydrolysis may cause coeliac sprue in genetically predisposed individuals with high expression of main histocompatibility complex HLA-DQ2 or DQ8, since by consuming wheat, they become exposed to proline- (P) and glutamine (Q)-rich gluten. In bread-making, the hydrolysis of gliadins and coeliac-toxic peptides occurs with varied efficiency depending on the fermentation pH and temperature. Degradation of gliadins catalysed by Lactobacillus acidophilus 5e2 peptidases and a commercial prolyl endopeptidase synthesised by A. niger, carried out at pH 4.0 and 37 ◦C, reduces the gliadin concentration over 110-fold and decreases the relative immunoreactivity of the hydrolysate to 0.9% of its initial value. Hydrolysis of coeliac-toxic peptides: LGQQQPFPPQQPY (P1) and PQPQLPYPQPQLP (P2) under the same conditions occurs with the highest efficiency, reaching 99.8 0.0% and 97.5 0.1%, respectively. ± ± The relative immunoreactivity of peptides P1 and P2 was 0.8 0.0% and 3.2 0.0%, respectively. ± ± A mixture of peptidases from L. acidophilus 5e2 and A. niger may be used in wheat sourdough fermentation to reduce the time needed for degradation of proteins and products of their hydrolysis. -

CDH12 Cadherin 12, Type 2 N-Cadherin 2 RPL5 Ribosomal
5 6 6 5 . 4 2 1 1 1 2 4 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 2 2 A A A A A A A A A A A A A A A A A A A A C C C C C C C C C C C C C C C C C C C C R R R R R R R R R R R R R R R R R R R R B , B B B B B B B B B B B B B B B B B B B , 9 , , , , 4 , , 3 0 , , , , , , , , 6 2 , , 5 , 0 8 6 4 , 7 5 7 0 2 8 9 1 3 3 3 1 1 7 5 0 4 1 4 0 7 1 0 2 0 6 7 8 0 2 5 7 8 0 3 8 5 4 9 0 1 0 8 8 3 5 6 7 4 7 9 5 2 1 1 8 2 2 1 7 9 6 2 1 7 1 1 0 4 5 3 5 8 9 1 0 0 4 2 5 0 8 1 4 1 6 9 0 0 6 3 6 9 1 0 9 0 3 8 1 3 5 6 3 6 0 4 2 6 1 0 1 2 1 9 9 7 9 5 7 1 5 8 9 8 8 2 1 9 9 1 1 1 9 6 9 8 9 7 8 4 5 8 8 6 4 8 1 1 2 8 6 2 7 9 8 3 5 4 3 2 1 7 9 5 3 1 3 2 1 2 9 5 1 1 1 1 1 1 5 9 5 3 2 6 3 4 1 3 1 1 4 1 4 1 7 1 3 4 3 2 7 6 4 2 7 2 1 2 1 5 1 6 3 5 6 1 3 6 4 7 1 6 5 1 1 4 1 6 1 7 6 4 7 e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m -

Proteolytic Enzymes in Grass Pollen and Their Relationship to Allergenic Proteins
Proteolytic Enzymes in Grass Pollen and their Relationship to Allergenic Proteins By Rohit G. Saldanha A thesis submitted in fulfilment of the requirements for the degree of Masters by Research Faculty of Medicine The University of New South Wales March 2005 TABLE OF CONTENTS TABLE OF CONTENTS 1 LIST OF FIGURES 6 LIST OF TABLES 8 LIST OF TABLES 8 ABBREVIATIONS 8 ACKNOWLEDGEMENTS 11 PUBLISHED WORK FROM THIS THESIS 12 ABSTRACT 13 1. ASTHMA AND SENSITISATION IN ALLERGIC DISEASES 14 1.1 Defining Asthma and its Clinical Presentation 14 1.2 Inflammatory Responses in Asthma 15 1.2.1 The Early Phase Response 15 1.2.2 The Late Phase Reaction 16 1.3 Effects of Airway Inflammation 16 1.3.1 Respiratory Epithelium 16 1.3.2 Airway Remodelling 17 1.4 Classification of Asthma 18 1.4.1 Extrinsic Asthma 19 1.4.2 Intrinsic Asthma 19 1.5 Prevalence of Asthma 20 1.6 Immunological Sensitisation 22 1.7 Antigen Presentation and development of T cell Responses. 22 1.8 Factors Influencing T cell Activation Responses 25 1.8.1 Co-Stimulatory Interactions 25 1.8.2 Cognate Cellular Interactions 26 1.8.3 Soluble Pro-inflammatory Factors 26 1.9 Intracellular Signalling Mechanisms Regulating T cell Differentiation 30 2 POLLEN ALLERGENS AND THEIR RELATIONSHIP TO PROTEOLYTIC ENZYMES 33 1 2.1 The Role of Pollen Allergens in Asthma 33 2.2 Environmental Factors influencing Pollen Exposure 33 2.3 Classification of Pollen Sources 35 2.3.1 Taxonomy of Pollen Sources 35 2.3.2 Cross-Reactivity between different Pollen Allergens 40 2.4 Classification of Pollen Allergens 41 2.4.1 -

Review Article the Role of Microbial Aspartic Protease Enzyme in Food and Beverage Industries
Hindawi Journal of Food Quality Volume 2018, Article ID 7957269, 15 pages https://doi.org/10.1155/2018/7957269 Review Article The Role of Microbial Aspartic Protease Enzyme in Food and Beverage Industries Jermen Mamo and Fassil Assefa Microbial, Cellular and Molecular Biology Department, College of Natural Science, Addis Ababa University, P.O. Box 1176, Addis Ababa, Ethiopia Correspondence should be addressed to Jermen Mamo; [email protected] Received 3 April 2018; Revised 16 May 2018; Accepted 29 May 2018; Published 3 July 2018 Academic Editor: Antimo Di Maro Copyright © 2018 Jermen Mamo and Fassil Assefa. is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Proteases represent one of the three largest groups of industrial enzymes and account for about 60% of the total global enzymes sale. According to the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology, proteases are classied in enzymes of class 3, the hydrolases, and the subclass 3.4, the peptide hydrolases or peptidase. Proteases are generally grouped into two main classes based on their site of action, that is, exopeptidases and endopeptidases. Protease has also been grouped into four classes based on their catalytic action: aspartic, cysteine, metallo, and serine proteases. However, lately, three new systems have been dened: the threonine-based proteasome system, the glutamate-glutamine system of eqolisin, and the serine-glutamate-aspartate system of sedolisin. Aspartic proteases (EC 3.4.23) are peptidases that display various activities and specicities. -

Treatment of Cashew Extracts with Aspergillopepsin Reduces Ige Binding to Cashew Allergens
Journal of Applied Biology & Biotechnology Vol. 4 (02), pp. 001-010, March-April, 2016 Available online at http://www.jabonline.in DOI: 10.7324/JABB.2016.40201 Treatment of cashew extracts with Aspergillopepsin reduces IgE binding to cashew allergens Cecily B. DeFreece1, Jeffrey W. Cary2, Casey C. Grimm2, Richard L. Wasserman3, Christopher P Mattison2* 1Department of Biology, Xavier University of Louisiana, New Orleans, LA, USA. 2USDA-ARS, Southern Regional Research Center, New Orleans, LA, USA. 3Allergy Partners of North Texas Research, Department of Pediatrics, Medical City Children’s Hospital, Dallas, Texas, USA. ARTICLE INFO ABSTRACT Article history: Enzymes from Aspergillus fungal species are used in many industrial and pharmaceutical applications. Received on: 21/10/2015 Aspergillus niger and Aspergillus oryzae were cultured on media containing cashew nut flour to identify secreted Revised on: 06/12/2015 proteins that may be useful as future food allergen processing enzymes. Mass-spectrometric analysis of secreted Accepted on: 20/12/2015 proteins and protein bands from SDS-PAGE gels indicated the presence of at least 63 proteins. The majority of Available online: 21/04/2016 these proteins were involved in carbohydrate metabolism, but there were also enzymes involved in lipid and protein metabolism. It is likely that some of these enzymes are specifically upregulated in response to cashew Key words: nut protein, and study of these enzymes could aid our understanding of cashew nut metabolism. Aspergillus, cashew, food Aspergillopepsin from A. niger was one of the proteolytic enzymes identified, and 6 distinct peptides were allergy, immunoglobulin E, matched to this protein providing 22% coverage of the protein. -

Trypsin-Like Proteases and Their Role in Muco-Obstructive Lung Diseases
International Journal of Molecular Sciences Review Trypsin-Like Proteases and Their Role in Muco-Obstructive Lung Diseases Emma L. Carroll 1,†, Mariarca Bailo 2,†, James A. Reihill 1 , Anne Crilly 2 , John C. Lockhart 2, Gary J. Litherland 2, Fionnuala T. Lundy 3 , Lorcan P. McGarvey 3, Mark A. Hollywood 4 and S. Lorraine Martin 1,* 1 School of Pharmacy, Queen’s University, Belfast BT9 7BL, UK; [email protected] (E.L.C.); [email protected] (J.A.R.) 2 Institute for Biomedical and Environmental Health Research, School of Health and Life Sciences, University of the West of Scotland, Paisley PA1 2BE, UK; [email protected] (M.B.); [email protected] (A.C.); [email protected] (J.C.L.); [email protected] (G.J.L.) 3 Wellcome-Wolfson Institute for Experimental Medicine, School of Medicine, Dentistry and Biomedical Sciences, Queen’s University, Belfast BT9 7BL, UK; [email protected] (F.T.L.); [email protected] (L.P.M.) 4 Smooth Muscle Research Centre, Dundalk Institute of Technology, A91 HRK2 Dundalk, Ireland; [email protected] * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: Trypsin-like proteases (TLPs) belong to a family of serine enzymes with primary substrate specificities for the basic residues, lysine and arginine, in the P1 position. Whilst initially perceived as soluble enzymes that are extracellularly secreted, a number of novel TLPs that are anchored in the cell membrane have since been discovered. Muco-obstructive lung diseases (MucOLDs) are Citation: Carroll, E.L.; Bailo, M.; characterised by the accumulation of hyper-concentrated mucus in the small airways, leading to Reihill, J.A.; Crilly, A.; Lockhart, J.C.; Litherland, G.J.; Lundy, F.T.; persistent inflammation, infection and dysregulated protease activity.