PET Coding Review and Resources
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PET/CT Evaluation of Cardiac Sarcoidosis
PET/CT Evaluation of Cardiac Sarcoidosis John P. Bois, MDa,*, Daniele Muser, MDb,1, Panithaya Chareonthaitawee, MDa KEYWORDS Cardiac sarcoidosis Positron emission tomography Fluorine-18 deoxyglucose KEY POINTS Sarcoidosis can involve the heart at with resultant significant morbidity and mortality. PET/CT is the most accurate method by which to diagnose cardiac sarcoidosis. Patient preparation prior to the PET/CT cardiac sarcoid study is critical to ensure diagnostic images are obtained. PET/CT detection of both active inflammation and scar has diagnostic, prognostic, and therapeutic importance. Ongoing areas of research include the use of PET to quantify the extent of myocardial inflammation and the discrepancies in myocardial blood flow in the cardiac sarcoidosis population. INTRODUCTION experiencing spontaneous remission and the remaining one-third developing either a stable or The increasing implementation of advanced car- progressive course.3 diovascular imaging in the form of cardiac PET/ The rate of cardiac involvement by sarcoidosis, CT has had a significant impact on the manage- otherwise termed CS, is variable and ranges ment of cardiac sarcoidosis (CS), one that con- from 20% to 75%.4,5 Furthermore, CS accounts tinues to evolve. Sarcoidosis is characterized for one-fourth of sarcoid-related mortality in the histologically by the presence of noncaseating United States and upward of 85% of death attrib- granulomas, with a predilection for the pulmonary uted to sarcoidosis in the Japanese population.4,6 system but with the ability to involve nearly every The high rate of involvement of the cardiovascular organ. Although the development of sarcoidosis system by sarcoidosis coupled with the potential is believed the sequelae of an exaggerated im- lethal outcomes has rendered accurate and timely mune or inflammatory response to an inciting in- diagnosis of this disease entity as imperative to fectious or environmental trigger, the specific patient care. -

Myocardial Perfusion Imaging with PET
SPECIAL CONTRIBUTION Myocardial Perfusion Imaging with PET Markus Schwaiger Nuklearmedizinische Klinik und Poliklini/çDer Technischen Universitdt Munchen, Klini/wm Rechts der Isar, Munich, Germany SPEC!' (5). PET spatial resolution is superior to that of AlthoughSPECThas become an acceptedimagingtechnique SPECT, resulting in superior image quality and less partial formyocardialperfusionstudies,thereare severaladvantages volume effect (5). Most PET images are processed with a to evaluatingcoronaryarterydisease (CAD)withPET.CADis a spatial resolution of about 6—10mm, as compared with complex, dynamic disease and quantitativemeasurements of 10—15mm for SPECF image reconstruction. myocardialbloodflowby PET can improvethe fUnCtiOnalchar The majoradvantageof PET is its ability to correct for acterizationof CAD.The majoradvantage of PET over SPECT attenuation.Traditionalmyocardialperfusionimagingwith is @rtsabilityto provideattenuation-correctedimages, whichde single-photon radiotracers such as 201'flposes significant creases incidenceof attenuation artifactsand increases spea challenges in avoiding or identifying and correcting for flcfty.Myocardialpertusion imagingwithPET can also provide moreaccurateinformationon localizationofdisease, as wellas attenuation artifacts, particularly those that involve the quantitativeassessment, inabsolutevalues, ofmyocardialblood inferior wall in male patients and the anterior wall in female flow.The measurement ofregionalflowreserve allowsforphys patients (6,7). iologiccharacterizationof stenosis severity, -

Time-Of-Flight PET Map out Goals by Joel S
Volume 3, Issue 4 FALL 2006 pet center of excellence newsletter PET COE Board Meets with Industry Advisory Group to Time-of-Flight PET Map Out Goals By Joel S. Karp, PhD he idea to use time-of-flight (TOF) information in PET image reconstruction By James W. Fletcher, MD Twas originally proposed in the 1960s at a very early stage in the development of President, PET Center of Excellence positron imaging. By the early 1980s, fully functional TOF PET systems had been built, An inaugural meet- not long after the first conventional PET systems were completed. Why then did it take ing was held recently so long to introduce a clinical TOF PET scanner, and how does it compare to the first in Chicago between the TOF PET instruments built 25 years ago? PET Center of Excel- Time-of-Flight Theory lence Board of Directors The concept of time-of-flight means simply that for each annihilation event, we note (BOD) and the Industry the precise time that each of the coincident photons is detected and calculate the dif- Advisory Group (IAG). ference. Since the closer photon will arrive at its detector first, the difference in arrival The meeting was very times helps pin down the location of the annihilation event along the line between the James W. Fletcher well attended with rep- two detectors. resentation from a large To understand why this information is useful, we need to recall that normally in cross-section of industry. PET we collect line pair data at many angles and create tomographic images through The interaction and discussion at the con- traditional filtered back-projection or through an iterative series of back- and forward- joint morning meeting was lively and infor- projection steps. -

SPR 2013 Postgraduate Course May 14-15, 2013 SAM Questionnaire Tuesday, May 14, 2013 CHEST Digital Radiography Robert Macdougal
SPR 2013 Postgraduate Course May 14-15, 2013 SAM Questionnaire Tuesday, May 14, 2013 CHEST Digital Radiography Robert MacDougall, MSc 1. Which of the following is unaffected by the selection of Value of Interest Look Up Table (VOI-LUT): A. Diagnostic information in the processed image B. Target Exposure (ET) C. Exposure Index (EI) D. Deviation Index (DI) E. Brightness and contrast of the displayed image Correct Answer: B 2. Exposure Index (EI) represents: A. The exposure at the entrance to the patient B. The exposure at the detector plane measured with an ion chamber C. The brightness of the displayed image D. The exposure at the detector calculated from the mean signal response of the detector within the Values of Interest E. The deviation from a target exposure Correct Answer: D References 1. An Exposure Indicator for Digital Radiography: Report of AAPM Task Group 116. American Association of Physicists in Medicine. Accessed April 10, 2013. https://www.aapm.org/pubs/reports/RPT_116.pdf 2. Internation Electrotechnical Commission. Medical Electrical Equipment. Exposure Index of Digital X-ray Imaging Systems - Part 1: Definitions and Requirements for General Radiography. IEC Publication No. 62494-1. Geneva, Switzerland: International Electrotechnical Commission, 2002. Functional Chest MR Imaging Hyun Woo Goo, MD, PhD 3. Which one of the followings is the LEAST likely limitation of thoracic MR imaging? A. Low signal-to-noise ratio due to the low proton density of the lung B. Potential hazards from ionizing radiation C. Motion artifacts from respiratory motion and cardiac pulsation D. Relatively long examination time E. Susceptibility artifacts from multiple air-tissue interfaces Correct Answer: B References 1. -
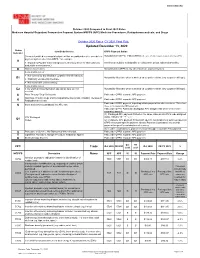
Updated December 13, 2020
WWW.SNMMI.ORG October 2020 Compared to Final 2021 Rates Medicare Hospital Outpatient Prospective Payment System HOPPS (APC) Medicine Procedures, Radiopharmaceuticals, and Drugs October 2020 Rates CY 2021 Final Rule Updated December 13, 2020 Status Item/Code/Service OPPS Payment Status Indicator Services furnished to a hospital outpatient that are paid under a fee schedule or Not paid under OPPS. Paid by MACs under a fee schedule or payment system other than OPPS. payment system other than OPPS,* for example: A ● Separately Payable Clinical Diagnostic Laboratory Services (Not subject to Services are subject to deductible or coinsurance unless indicated otherwise. deductible or coinsurance.) D Discontinued Codes Not paid under OPPS or any other Medicare payment system. Items and Services: ● Not covered by any Medicare outpatient benefit category Not paid by Medicare when submitted on outpatient claims (any outpatient bill type). E1 ● Statutorily excluded by Medicare ● Not reasonable and necessary Items and Services: E2 ● for which pricing information and claims data are not Not paid by Medicare when submitted on outpatient claims (any outpatient bill type). available G Pass-Through Drug/ Biologicals Paid under OPPS; separate APC payment NonPass-Through Drugs and nonimplantable Biologicals, including Therapeutic Paid under OPPS; separate APC payment K Radiopharmaceuticals Paid under OPPS; payment is packaged into payment for other services. Therefore, Items and Services packaged into APC rate N there is no separate APC payment. Paid under OPPS; Addendum B displays APC assignments when services are separately payable. (1) Packaged APC payment if billed on the same claim as a HCPCS code assigned STV-Packaged status indicator “S,” “T,” or “V.” Q1 Codes (2) Composite APC payment if billed with specific combinations of services based on OPPS composite-specific payment criteria. -

Atrium Health Delineation of Privileges Specialty of Radiology
ATRIUM HEALTH DELINEATION OF PRIVILEGES SPECIALTY OF RADIOLOGY Print Name YES NO** I have participated in direct patient care in the hospital setting within the past two (2) years. **If the answer is No, please do not complete this form. Contact the Medical Staff Office at (704) 355-2147 for additional instructions regarding the required proctoring process. Initial appointment Reappointment Updated DOP Request for Clinical Privileges To be eligible for core privileges in Radiology, the applicant must meet the following qualifications: If the applicant is not currently certified in Radiology by the American Board of Medical Specialties (ABMS) or the American Osteopathic Association (AOA) the applicant must: 1. Provide documentation of successful completion of an ACGME or AOA accredited Radiology training program, within the past five (5) years; AND 2. Provide documentation of the performance and interpretation of at least five-thousand (5,000) imaging tests in the past two (2) years. Applicants have the burden of producing information deemed adequate by the hospital for proper evaluation of current competence, and other qualifications and for resolving any doubts; OR If the applicant is currently certified in Radiology by the American Board of Medical Specialties (ABMS) or the American Osteopathic Association (AOA), the applicant must: 1. Provide documentation of general pediatric certification from the American Board of Medical Specialties or the American Osteopathic Association (AOA); AND 2. Provide documentation of the performance and interpretation of at least five-thousand (5,000) imaging tests in the past two (2) years. Applicants have the burden of producing information deemed adequate by the hospital for proper evaluation of current competence, and other qualifications and for resolving any doubts. -

Cardiac Radiology)
CAE002-b F-18 FDG PET/CT and MRI In the Diagnosis and Management of Cardiac Sarcoidosis Education Exhibits Location: CA Community, Learning Center Participants Richard Anthony R. Coulden MD (Presenter): Nothing to Disclose Emer Sonnex : Nothing to Disclose Hefin Jones FRCR : Nothing to Disclose Indrajeet Das MBBCh, MRCP : Nothing to Disclose Jonathan Thomas Abele MD : Nothing to Disclose TEACHING POINTS In patients with established non-cardiac sarcoidosis, both FDG PET/CT and cardiac MRI can be used to diagnose cardiac involvement. We will learn how and why: 1. FDG PET/CT identifies active disease and can be used in both diagnosis and management. Serial PET allows assessment of response to immunosuppressive treatment. 2. Cardiac MRI identifies myocardial edema and scar. It has proven value in diagnosis but its role in monitoring disease in response to treatment is unclear. 3. Cardiac MRI provides additional value in assessment of ventricular volumes and function and maybe a helpful surrogate in monitoring treatment response. 4. FDG PET/CT and MRI are complementary techniques. TABLE OF CONTENTS/OUTLINE 1. Criteria for clinical diagnosis of cardiac sarcoidosis (Japanese Ministry of Health and Welfare) 2. How to use FDG PET/CT for inflammatory cardiac imaging 3. How to use cardiac MRI for infiltrative cardiomyopathies 4. Relative roles of Cardiac MRI and FDG PET/CT in: a. the imaging diagnosis of cardiac sarcoidosis b. follow-up of disease activity and response to immunosuppressive treatment. CAE004-b Dynamic Myocardial Perfusion Imaging by 3rd Generation Dual-Source CT Education Exhibits Location: CA Community, Learning Center Participants Marisa Marjolein Lubbers MD (Presenter): Nothing to Disclose Adriaan Coenen MD : Nothing to Disclose Akira Kurata : Nothing to Disclose Marcel L. -

Criteria for Acceptability of Medical Radiological Equipment Used in Diagnostic Radiology, Nuclear Medicine and Radiotherapy
EUROPEAN COMMISSION RADIATION PROTECTION N° 162 Criteria for Acceptability of Medical Radiological Equipment used in Diagnostic Radiology, Nuclear Medicine and Radiotherapy Directorate-General for Energy Directorate D — Nuclear Safety & Fuel Cycle Unit D4 — Radiation Protection 2012 This report was prepared by Quality Assurance Reference Centre for the European Commission under contract N°. ENER/10/NUCL/SI2.581655 and represents those organisations’ views on the subject matter. The views and opinions expressed herein do not necessarily state or reflect those of the European Commission and should not be relied upon as a statement of the Commission’s views. The European Commission does not guarantee the accuracy of the data included in this report, nor does it accept responsibility for any use made thereof. Europe Direct is a service to help you find answers to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00 800 numbers or these calls may be billed. More information on the European Union is available on the Internet (http://europa.eu). Luxembourg: Publications Office of the European Union, 2012 ISBN 978-92-79-27747-4 doi: 10.2768/22561 © European Union, 2012 Reproduction is authorised provided the source is acknowledged. Printed in Luxembourg 2 FOREWORD Luxembourg, October 2012 The work of the European Commission in the field of radiation protection is governed by the Euratom Treaty and the secondary legislation adopted under it. Council Directive 97/43/Euratom (the Medical Exposure Directive, MED) is the legal act defining the Euratom requirements on radiation protection of patients and of other individuals submitted to medical exposure. -

Diagnostic Radiology Physics Diagnostic This Publication Provides a Comprehensive Review of Topics Relevant to Diagnostic Radiology Physics
A Handbook for Teachers and Students A Handbook for Teachers Diagnostic Diagnostic This publication provides a comprehensive review of topics relevant to diagnostic radiology physics. It is intended to provide the basis for the education of medical physicists in the field of diagnostic radiology. Bringing together the work of 41 authors and reviewers from 12 countries, the handbook covers a broad range of topics including radiation physics, dosimetry and Radiology instrumentation, image quality and image perception, imaging modality specific topics, recent advances in digital techniques, and radiation biology and protection. It is not designed to replace the large number of textbooks available on many aspects of diagnostic radiology physics, but is expected Radiology Physics Physics to fill a gap in the teaching material for medical radiation physics in imaging, providing in a single manageable volume the broadest coverage of topics currently available. The handbook has been endorsed by several international professional bodies and will be of value to those preparing for their certification A Handbook for as medical physicists, radiologists and diagnostic radiographers. Teachers and Students D.R. Dance S. Christofides A.D.A. Maidment I.D. McLean K.H. Ng Technical Editors International Atomic Energy Agency Vienna ISBN 978–92–0–131010–1 1 @ DIAGNOSTIC RADIOLOGY PHYSICS: A HANDBOOK FOR TEACHERS AND STUDENTS The following States are Members of the International Atomic Energy Agency: AFGHANISTAN GHANA OMAN ALBANIA GREECE PAKISTAN ALGERIA GUATEMALA -

Evicore Cardiac Imaging Guidelines
CLINICAL GUIDELINES Cardiac Imaging Policy Version 1.0 Effective February 14, 2020 eviCore healthcare Clinical Decision Support Tool Diagnostic Strategies: This tool addresses common symptoms and symptom complexes. Imaging requests for individuals with atypical symptoms or clinical presentations that are not specifically addressed will require physician review. Consultation with the referring physician, specialist and/or individual’s Primary Care Physician (PCP) may provide additional insight. CPT® (Current Procedural Terminology) is a registered trademark of the American Medical Association (AMA). CPT® five digit codes, nomenclature and other data are copyright 2017 American Medical Association. All Rights Reserved. No fee schedules, basic units, relative values or related listings are included in the CPT® book. AMA does not directly or indirectly practice medicine or dispense medical services. AMA assumes no liability for the data contained herein or not contained herein. © 2019 eviCore healthcare. All rights reserved. Cardiac Imaging Guidelines V1.0 Cardiac Imaging Guidelines Abbreviations for Cardiac Imaging Guidelines 3 Glossary 4 CD-1: General Guidelines 5 CD-2: Echocardiography (ECHO) 15 CD-3: Nuclear Cardiac Imaging 26 CD-4: Cardiac CT, Coronary CTA, and CT for Coronary Calcium (CAC) 33 CD-5: Cardiac MRI 40 CD-6: Cardiac PET 45 CD-7: Diagnostic Heart Catheterization 49 CD-8: Pulmonary Artery and Vein Imaging 56 CD-9: Congestive Heart Failure 59 CD-10: Cardiac Trauma 62 CD-11: Adult Congenital Heart Disease 64 CD-12: Cancer Therapeutics-Related -

Myocardial Perfusion Imaging Versus CT Coronary Angiography: When to Use Which?
CONTINUING EDUCATION Myocardial Perfusion Imaging Versus CT Coronary Angiography: When to Use Which? Balaji Tamarappoo and Rory Hachamovitch Section of Cardiovascular Imaging, Department of Cardiovascular Medicine, Cleveland Clinic, Cleveland, Ohio Learning Objectives: On successful completion of this activity, participants should be able to describe (1) the various types of noninvasive cardiac imaging tests and how the results of each are utilized; (2) recent advances in CT coronary angiography, PET myocardial perfusion imaging, and SPECT myocardial perfusion imaging of coronary artery disease; and (3) the clinical use of anatomic versus physiologic tests in symptomatic patients with known or suspected coronary artery disease. Financial Disclosure: The authors of this article have indicated no relevant relationships that could be perceived as a real or apparent conflict of interest. CME Credit: SNM is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNM designates each JNM continuing education article for a maximum of 1.0 AMA PRA Category 1 Credit. Physicians should claim only credit commensurate with the extent of their participation in the activity. For CE credit, participants can access this activity through the SNM Web site (http://www.snm.org/ce_online) through June 2012. suspected coronary artery disease (CAD). The results of Both anatomy- and physiology-based approaches to patient these tests are used for patient risk stratification, evaluation management have advantages and limitations. Compared of myocardial ischemia as a cause of symptoms, and assess- with the latter, the former has a superior ability to exclude ment of ongoing disease management. Stress myocardial disease and does not miss high-risk coronary artery disease (CAD). -

Diffraction-Enhanced X-Ray Imaging of in Vitro Breast Tumours
UNIVERSITY OF HELSINKI REPORT SERIES IN PHYSICS HU-P-D113 DIFFRACTION-ENHANCED X-RAY IMAGING OF IN VITRO BREAST TUMOURS Jani Keyriläinen Division of X-ray Physics Department of Physical Sciences Faculty of Science University of Helsinki Helsinki, Finland Department of Oncology Helsinki University Central Hospital Helsinki, Finland ACADEMIC DISSERTATION To be presented, with the permission of the Faculty of Science of the University of Helsinki, for public criticism in Auditorium D101 of the Department of Physical Sciences (Physicum), Gustaf Hällströmin katu 2, on October 29th, 2004, at 12 o’clock noon. Helsinki 2004 ISSN 0356-0961 ISBN 952-10-1655-8 ISBN 952-10-1656-6 (pdf-version) http://ethesis.helsinki.fi/ Helsinki 2004 Yliopistopaino PREFACE This thesis is based on research done at the Division of X-ray Physics, Department of Physical Sciences, University of Helsinki (HU, Finland), at the Medical Beamline ID17, European Synchrotron Radiation Facility (ESRF, Grenoble, France), and at the departments of Oncology, Pathology and Radiology, Helsinki University Central Hospital (HUCH, Finland), all of which are acknowledged. I wish to express my gratitude to Professor Juhani Keinonen, Ph.D., Head of the Department of Physical Sciences, and to Professor Seppo Manninen, Ph.D., former Head of the Division of X-ray Physics, for the opportunity to work at the Department. I also wish to thank Professor Heikki Joensuu, M.D., Ph.D., Head of the Department of Oncology, and William Thomlinson, Ph.D., former Beamline Responsible, ID17, for allowing me to use the outstanding working facilities of their institutions. I am most grateful to my supervisors, Professor Pekka Suortti, Ph.D., Department of Physical Sciences, and Docent Mikko Tenhunen, Ph.D., Chief Physicist of the Department of Oncology, for proposing to me the topic of this study and guiding me throughout this research work.