Diffraction-Enhanced X-Ray Imaging of in Vitro Breast Tumours
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2. Screening Techniques
2. SCREENING TECHNIQUES 2.1 X-ray techniques the contrast and the spatial resolution are poor, making detection of small lesions difficult. The The original technique for mammography mammogram in Fig. 2.1b, from the same era, is of was introduced by Salomon in Germany in 1913, much higher quality and illustrates a cancer seen 18 years after the discovery of X-rays by Roentgen on the basis of an irregularly shaped mass (black (Salomon, 1913). A mammogram is formed by arrow). Fig. 2.1c shows a digital mammogram, recording the two-dimensional (2D) pattern of illustrating the enormous improvement that X-rays transmitted through the volume of the has occurred in both technology and technique. breast onto an image receptor. Breast cancer is Breast positioning, penetration of the tissue, and detected radiographically on the basis of four contrast are excellent, allowing visualization of major signs: a mass density with specific shape a small area of ductal carcinoma in situ (DCIS) and border characteristics, microcalcifications, seen on the basis of microcalcifications, and, architectural distortions, and asymmetries more importantly, providing the opportunity to between the radiological appearance of the left detect an immediately adjacent high-grade inva- and right breast (Kopans, 2006). These signs sive cancer 1.7 mm in diameter. are often very subtle, and in order for them to Excellent image quality is an essential compo- be detected accurately and when the cancer is at nent but not, on its own, a sufficient component the smallest detectable size, the technical image to ensure a high level of accuracy in cancer detec- quality of the mammograms must be excellent tion. -

Radiation Protection Guidance for Diagnostic X Rays
Disclaimer - For assistance accessing this document or additional information, please contact [email protected]. EPA 520/4-76-019 FEDERAL GUIDANCE REPORT NO. 9 RADIATION PROTECTION GUIDANCE FOR DIAGNOSTIC X RAYS ENVIRONMENTAL PROTECTION AGENCY INTERAGENCY WORKING GROUP ON MEDICAL RADIATION FEDERAL GUIDANCE REPORT NO. 9 RADIATION PROTECTION GUIDANCE FOR DIAGNOSTIC X RAYS Interagency Working Group on Medical Radiation U.S. Environmental Protection Agency Washington, D.C. 20460 October 1976 PREFACE The authority of the Federal Radiation Council to provide radiation protection guidance was transferred to the Environmental Protection Agency on December 2, 1970, by Reorganization Plan No. 3. Prior to this transfer, the Federal Radiation Council developed reports which provided the basis for guidance recommended to the President for use by Federal agencies in developing standards for a wide range of radiation exposure circumstances. This report, which was prepared in cooperation with an Interagency Working Group on Medical Radiation formed on July 5, 1974, constitutes a similar objective to provide the basis for recommendations to reduce unnecessary radiation exposure due to medical uses of diagnostic x rays. The Interagency Working Group developed its recommendations with the help of two subcommittees. The Subcommittee on Prescription of Exposure to X rays examined factors to eliminate clinically unproductive examinations and the Subcommittee on Technic of Exposure Prevention examined factors to assure the use of optimal technic in performing x-ray examinations. Both subcommittees also considered the importance of appropriate and properly functioning equipment in producing radiographs of the required diagnostic quality with minimal exposure. Reports by these subcommittees were made available for public comment. -

Amorphous Lead Oxide (A-Pbo): Suppression of Signal Lag Via Engineering of the Layer Structure Received: 12 June 2017 O
www.nature.com/scientificreports OPEN Amorphous lead oxide (a-PbO): suppression of signal lag via engineering of the layer structure Received: 12 June 2017 O. Semeniuk1,2, O. Grynko1,2, G. Juska3 & A. Reznik2,4 Accepted: 25 September 2017 Presence of a signal lag is a bottle neck of performance for many non-crystalline materials, considered Published: xx xx xxxx for dynamic radiation sensing. Due to inadequate lag-related temporal performance, polycrystalline layers of CdZnTe, PbI2, HgI2 and PbO are not practically utilized, despite their superior X-ray sensitivity and low production cost (even for large area detectors). In the current manuscript, we show that a technological step to replace nonhomogeneous disorder in polycrystalline PbO with homogeneous amorphous PbO structure suppresses signal lag and improves time response to X-ray irradiation. In addition, the newly developed amorphous lead oxide (a-PbO) possesses superior X-ray sensitivity in terms of electron-hole pair creation energy W± in comparison with amorphous selenium – currently the only photoconductor used as an X-ray-to-charge transducer in the state-of-the-art direct conversion X-ray medical imaging systems. The proposed advances of the deposition process are low cost, easy to implement and with certain customization might potentially be applied to other materials, thus paving the way to their wide-range commercial use. Amorphous and polycrystalline modifcations of wide band gap semiconductors are of paramount importance in modern electronics, since they allow large device area production at low cost. However, the transition from crystalline to non-crystalline materials is technologically challenging since structural disorder may lead to degra- dation of the material performance. -

Radiographic Diagnostic Aids: a Review © 2019 IJADS Received: 01-02-2019 Dr
International Journal of Applied Dental Sciences 2019; 5(2): 271-276 ISSN Print: 2394-7489 ISSN Online: 2394-7497 IJADS 2019; 5(2): 271-276 Radiographic diagnostic Aids: A review © 2019 IJADS www.oraljournal.com Received: 01-02-2019 Dr. Panna Mangat, Dr. Anil K Tomer, Dr. Afnan Ajaz Raina, Dr. Faizan Accepted: 03-03-2019 Bin Ayub, Dr. Akankshita Behera, Dr. Nitish Mittal, Dr. Megna Bhatt Dr. Panna Mangat Professor, Department of Conservative and Dr. Ayush Tyagi Dentistry & Endodontics D.J. College of Dental Sciences and Research, Modinagar, Ghaziabad, Uttar Pradesh, Abstract India Presently diagnosis has shown a major growth in the field of Endodontics. Newer technologies have evolved in a way that human elements are being enriched in a much better way to ensure proper and Dr. Anil K Tomer Professor and Head, Department of correct diagnosis. Therefore, for a successful diagnostician, a necessity arises to keep abreast of all the Conservative Dentistry & Endodontics new methods for correct diagnosis and treatment. The aim of this review therefore is to assess the D.J. College of Dental Sciences and usefulness of some radiographic diagnostic aids and techniques used in endodontic therapy to make the Research, Modinagar, Ghaziabad, Uttar correct pulpal diagnosis. Pradesh, India Dr. Afnan Ajaz Raina Keywords: Radiographic diagnostic, aids, endodontics Post Graduate Student, Department of Conservative Dentistry & Endodontics D.J. College of Dental Sciences and Introduction Research, Modinagar, Ghaziabad, Uttar Pradesh, India Diagnosis is arguably the most critical component of all dental treatment, and Endodontics is no exception. Stedman’s Medical Dictionary describes clinical diagnosis as ‘‘the Dr. -

SPR 2013 Postgraduate Course May 14-15, 2013 SAM Questionnaire Tuesday, May 14, 2013 CHEST Digital Radiography Robert Macdougal
SPR 2013 Postgraduate Course May 14-15, 2013 SAM Questionnaire Tuesday, May 14, 2013 CHEST Digital Radiography Robert MacDougall, MSc 1. Which of the following is unaffected by the selection of Value of Interest Look Up Table (VOI-LUT): A. Diagnostic information in the processed image B. Target Exposure (ET) C. Exposure Index (EI) D. Deviation Index (DI) E. Brightness and contrast of the displayed image Correct Answer: B 2. Exposure Index (EI) represents: A. The exposure at the entrance to the patient B. The exposure at the detector plane measured with an ion chamber C. The brightness of the displayed image D. The exposure at the detector calculated from the mean signal response of the detector within the Values of Interest E. The deviation from a target exposure Correct Answer: D References 1. An Exposure Indicator for Digital Radiography: Report of AAPM Task Group 116. American Association of Physicists in Medicine. Accessed April 10, 2013. https://www.aapm.org/pubs/reports/RPT_116.pdf 2. Internation Electrotechnical Commission. Medical Electrical Equipment. Exposure Index of Digital X-ray Imaging Systems - Part 1: Definitions and Requirements for General Radiography. IEC Publication No. 62494-1. Geneva, Switzerland: International Electrotechnical Commission, 2002. Functional Chest MR Imaging Hyun Woo Goo, MD, PhD 3. Which one of the followings is the LEAST likely limitation of thoracic MR imaging? A. Low signal-to-noise ratio due to the low proton density of the lung B. Potential hazards from ionizing radiation C. Motion artifacts from respiratory motion and cardiac pulsation D. Relatively long examination time E. Susceptibility artifacts from multiple air-tissue interfaces Correct Answer: B References 1. -
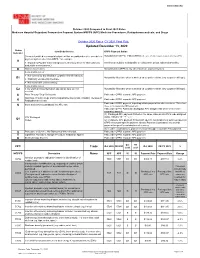
Updated December 13, 2020
WWW.SNMMI.ORG October 2020 Compared to Final 2021 Rates Medicare Hospital Outpatient Prospective Payment System HOPPS (APC) Medicine Procedures, Radiopharmaceuticals, and Drugs October 2020 Rates CY 2021 Final Rule Updated December 13, 2020 Status Item/Code/Service OPPS Payment Status Indicator Services furnished to a hospital outpatient that are paid under a fee schedule or Not paid under OPPS. Paid by MACs under a fee schedule or payment system other than OPPS. payment system other than OPPS,* for example: A ● Separately Payable Clinical Diagnostic Laboratory Services (Not subject to Services are subject to deductible or coinsurance unless indicated otherwise. deductible or coinsurance.) D Discontinued Codes Not paid under OPPS or any other Medicare payment system. Items and Services: ● Not covered by any Medicare outpatient benefit category Not paid by Medicare when submitted on outpatient claims (any outpatient bill type). E1 ● Statutorily excluded by Medicare ● Not reasonable and necessary Items and Services: E2 ● for which pricing information and claims data are not Not paid by Medicare when submitted on outpatient claims (any outpatient bill type). available G Pass-Through Drug/ Biologicals Paid under OPPS; separate APC payment NonPass-Through Drugs and nonimplantable Biologicals, including Therapeutic Paid under OPPS; separate APC payment K Radiopharmaceuticals Paid under OPPS; payment is packaged into payment for other services. Therefore, Items and Services packaged into APC rate N there is no separate APC payment. Paid under OPPS; Addendum B displays APC assignments when services are separately payable. (1) Packaged APC payment if billed on the same claim as a HCPCS code assigned STV-Packaged status indicator “S,” “T,” or “V.” Q1 Codes (2) Composite APC payment if billed with specific combinations of services based on OPPS composite-specific payment criteria. -

Criteria for Acceptability of Medical Radiological Equipment Used in Diagnostic Radiology, Nuclear Medicine and Radiotherapy
EUROPEAN COMMISSION RADIATION PROTECTION N° 162 Criteria for Acceptability of Medical Radiological Equipment used in Diagnostic Radiology, Nuclear Medicine and Radiotherapy Directorate-General for Energy Directorate D — Nuclear Safety & Fuel Cycle Unit D4 — Radiation Protection 2012 This report was prepared by Quality Assurance Reference Centre for the European Commission under contract N°. ENER/10/NUCL/SI2.581655 and represents those organisations’ views on the subject matter. The views and opinions expressed herein do not necessarily state or reflect those of the European Commission and should not be relied upon as a statement of the Commission’s views. The European Commission does not guarantee the accuracy of the data included in this report, nor does it accept responsibility for any use made thereof. Europe Direct is a service to help you find answers to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00 800 numbers or these calls may be billed. More information on the European Union is available on the Internet (http://europa.eu). Luxembourg: Publications Office of the European Union, 2012 ISBN 978-92-79-27747-4 doi: 10.2768/22561 © European Union, 2012 Reproduction is authorised provided the source is acknowledged. Printed in Luxembourg 2 FOREWORD Luxembourg, October 2012 The work of the European Commission in the field of radiation protection is governed by the Euratom Treaty and the secondary legislation adopted under it. Council Directive 97/43/Euratom (the Medical Exposure Directive, MED) is the legal act defining the Euratom requirements on radiation protection of patients and of other individuals submitted to medical exposure. -

Diagnostic Radiology Physics Diagnostic This Publication Provides a Comprehensive Review of Topics Relevant to Diagnostic Radiology Physics
A Handbook for Teachers and Students A Handbook for Teachers Diagnostic Diagnostic This publication provides a comprehensive review of topics relevant to diagnostic radiology physics. It is intended to provide the basis for the education of medical physicists in the field of diagnostic radiology. Bringing together the work of 41 authors and reviewers from 12 countries, the handbook covers a broad range of topics including radiation physics, dosimetry and Radiology instrumentation, image quality and image perception, imaging modality specific topics, recent advances in digital techniques, and radiation biology and protection. It is not designed to replace the large number of textbooks available on many aspects of diagnostic radiology physics, but is expected Radiology Physics Physics to fill a gap in the teaching material for medical radiation physics in imaging, providing in a single manageable volume the broadest coverage of topics currently available. The handbook has been endorsed by several international professional bodies and will be of value to those preparing for their certification A Handbook for as medical physicists, radiologists and diagnostic radiographers. Teachers and Students D.R. Dance S. Christofides A.D.A. Maidment I.D. McLean K.H. Ng Technical Editors International Atomic Energy Agency Vienna ISBN 978–92–0–131010–1 1 @ DIAGNOSTIC RADIOLOGY PHYSICS: A HANDBOOK FOR TEACHERS AND STUDENTS The following States are Members of the International Atomic Energy Agency: AFGHANISTAN GHANA OMAN ALBANIA GREECE PAKISTAN ALGERIA GUATEMALA -

Report of the Working Group on Digital Mammography: Digital Displays and Workstation Design
Report of the Working Group on Digital Mammography: Digital Displays and Workstation Design March 9-10, 1998 Washington, DC Sponsored by Public Health Service’s Office on Women’s Health and National Cancer Institute Editors Faina Shtern, M.D. Daniel Winfield, M.S. USPHS—Office on Women’s Health Research Triangle Institute Contributors Fred Behlen, Ph.D. Elizabeth A. Krupinski, Ph.D. University of Chicago University of Arizona Hartwig Blume, Ph.D. Harold Kundel, M.D. Phillips Medical Systems University of Pennsylvania Medical Center Michael J. Flynn, Ph.D. Hans Roehrig, Ph.D. Henry Ford Health System University of Arizona Bradley Hemminger, Ph.D. Peter E. Shile, M.D. University of North Carolina Mallinckrodt Institute of Radiology H. K. Huang, D.Sc. Edward Sickles, M.D. University of California at San Francisco University of California at San Francisco Martin Yaffe, Ph.D., M.Sc. University of Toronto Acknowledgments Daniel Sullivan, M.D. Wanda K. Jones, Dr.P.H. Robert E. Wittes, M.D. National Cancer Institute USPHS—Office on Women’s Health National Cancer Institute Contents Speakers and Panelists........................................................................................................................................................ii Introduction..........................................................................................................................................................................1 Goals of the Joint PHS OWH/NCI Working Group..........................................................................................................2 -

Whole Body MR
Whole Body MR: Techniques and Staging in Oncology ‐ How To • Extent of disease and staging • Response to treatment – Early assessment of response to treatment may allow more individualized therapy • Surveillance • Complications – Osteonecrosis – Infection • Cancer predisposition syndromes screening Condition Associated neoplasms Surveillance NF type I Optic nerve glioma, neurofibromas, leukemia (especially juvenile Annual physical examination; annual ophthalmologic examination in early childhood (to age 5 y); regular myelomonocytic leukemia developmental assessment and blood pressure monitoring; appropriate monitoring by a specialist and myelodysplastic syndromes, MPNST (lifetime risk of 8%–13%), GIST according to CNS, skeletal, or cardiovascular abnormalities (lifetime risk of 6%), pheochromocytoma (1%), rhabdomyosarcoma, neuroblastoma Beckwith- Wilms tu (40%–43%), hepatoblastoma (12-20%), adrenocortical ca, Abdominal US every 3 mo to age 7 y; measurement of serum AFP level every 3 mo to age 4 y; daily Wiedemann neuroblastoma, rhabdomyosarcoma abdominal examination by the caretaker at the discretion of the caretaker or parent; abdominal syndrome examination by a physician every 6 mo MEN 1 Parathyroid gland adenomas (65%–90%), pancreatic neuroendocrine tumors Screening starting at age 5–10 y, including measurement of fasting glucose, calcium, PTH, insulin, (50%–70%), and anterior pituitary gland adenomas (25%–65%) prolactin, and IGF1 levels; annual pancreatic US; pancreatic and pituitary MR imaging every 3–5 y; yearly abdominal CT or MR -

Xeroradiography – Review Article Dr
European Journal of Molecular & Clinical Medicine ISSN 2515-8260 Volume 07, Issue 5, 2020 Xeroradiography – Review Article Dr. R. Sankarnarayanan1, Dr. J. Amritha2 1. Reader, Dept. of Oral medicine and Radiology, Sree Balaji Dental College and Hospital, Bharath Institute of Higher Education and Research, Chennai. 2. Undergraduate Student, Sree Balaji Dental College and Hospital, Bharath Institute of Higher Education and Research, Chennai. Mail id: [email protected] ABSTRACT: Xeroradiography is a highly accurate electrostatic imaging technique. In this technique a conventional single‐phase dental x‐ray unit is used as an x‐ray source, but instead of a silver‐halide film image, a uniformly charged selenium alloy plate housed in a light‐proof cassette is used. It is used for detecting carious lesions, calculus deposits, periodontal disease and interpreting periapical structures. Keywords: Oral Medicine, Xeroradiography, Selenium. INTRODUCTION: Xeroradiography is the production of a visible image utilising the charged surface of a photoconductor (amorphous selenium) as the detecting medium, partially dissipating the charge by exposure to x-ray to form a latent image and making the latent image visible by xerographic processing. [1][2]. Xeroradiography which is a method of imaging uses the xeroradiographic copying process to record images produced by diagnostic x-rays.[1] It differs from halide film technique in that it involves neither wet chemical processing nor the use of dark room.[1] There are several features of xeroradiography that makes it as attractive imaging system in specific diagnostic situations, like: 1. Pronounced edge enhancement. 2. High contrast. 3. A choice of positive and negative displays. 4. Good detail. -

Whole Body Low Dose Computed Tomography (WBLDCT) Can Be Comparable to Whole-Body Magnetic Resonance Imaging (WBMRI) in the Assessment of Multiple Myeloma
diagnostics Article Whole Body Low Dose Computed Tomography (WBLDCT) Can Be Comparable to Whole-Body Magnetic Resonance Imaging (WBMRI) in the Assessment of Multiple Myeloma Davide Ippolito 1,2,* , Teresa Giandola 1,2, Cesare Maino 1,2 , Davide Gandola 1,2 , Maria Ragusi 1,2, Pietro Andrea Bonaffini 2,3 and Sandro Sironi 2,3 1 Department of Diagnostic Radiology, “San Gerardo” Hospital, via Pergolesi 33, 20900 Monza, MB, Italy; [email protected] (T.G.); [email protected] (C.M.); [email protected] (D.G.); [email protected] (M.R.) 2 School of Medicine, University of Milano-Bicocca, via Cadore 48, 20900 Monza, MB, Italy; pa.bonaffi[email protected] (P.A.B.); [email protected] (S.S.) 3 Department of Diagnostic Radiology, H Papa Giovanni XXIII, Piazza OMS 1, 24127 Bergamo, BG, Italy * Correspondence: [email protected] Abstract: Aim of the study is to compare the agreement between whole-body low-dose computed tomography (WBLDCT) and magnetic resonance imaging (WBMRI) in the evaluation of bone marrow involvement in patients with multiple myeloma (MM). Patients with biopsy-proven MM, who underwent both WBLDCT and WBMRI were retrospectively enrolled. After identifying the presence of focal bone involvement (focal infiltration pattern), the whole skeleton was divided into five Citation: Ippolito, D.; Giandola, T.; anatomic districts (skull, spine, sternum and ribs, pelvis, and limbs). Patients were grouped according Maino, C.; Gandola, D.; Ragusi, M.; to the number and location of the lytic lesions (<5, 5–20, and >20) and Durie and Salmon staging Bonaffini, P.A.; Sironi, S. Whole Body system. The agreement between CT and MRI regarding focal pattern, staging, lesion number, and Low Dose Computed Tomography distribution was assessed using the Cohen Kappa statistics.