Parts of the Nerve Cell and Their Functions 1. Cell Body
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
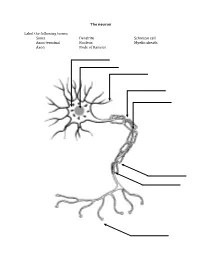
The Neuron Label the Following Terms: Soma Axon Terminal Axon Dendrite
The neuron Label the following terms: Soma Dendrite Schwaan cell Axon terminal Nucleus Myelin sheath Axon Node of Ranvier Neuron Vocabulary You must know the definitions of these terms 1. Synaptic Cleft 2. Neuron 3. Impulse 4. Sensory Neuron 5. Motor Neuron 6. Interneuron 7. Body (Soma) 8. Dendrite 9. Axon 10. Action Potential 11. Myelin Sheath (Myelin) 12. Afferent Neuron 13. Threshold 14. Neurotransmitter 15. Efferent Neurons 16. Axon Terminal 17. Stimulus 18. Refractory Period 19. Schwann 20. Nodes of Ranvier 21. Acetylcholine STEPS IN THE ACTION POTENTIAL 1. The presynaptic neuron sends neurotransmitters to postsynaptic neuron. 2. Neurotransmitters bind to receptors on the postsynaptic cell. - This action will either excite or inhibit the postsynaptic cell. - The soma becomes more positive. 3. The positive charge reaches the axon hillock. - Once the threshold of excitation is reached the neuron will fire an action potential. 4. Na+ channels open and Na+ is forced into the cell by the concentration gradient and the electrical gradient. - The neuron begins to depolarize. 5. The K+ channels open and K+ is forced out of the cell by the concentration gradient and the electrical gradient. - The neuron is depolarized. 6. The Na+ channels close at the peak of the action potential. - The neuron starts to repolarize. 7. The K+ channels close, but they close slowly and K+ leaks out. 8. The terminal buttons release neurotransmitter to the postsynaptic neuron. 9. The resting potential is overshot and the neuron falls to a -90mV (hyperpolarizes). - The neuron continues to repolarize. 10. The neuron returns to resting potential. The Synapse Label the following terms: Pre-synaptic membrane Neurotransmitters Post-synaptic membrane Synaptic cleft Vesicle Post-synaptic receptors . -

Crayfish Escape Behavior and Central Synapses
Crayfish Escape Behavior and Central Synapses. III. Electrical Junctions and Dendrite Spikes in Fast Flexor Motoneurons ROBERT S. ZUCKER Department of Biological Sciences and Program in the Neurological Sciences, Stanford University, Stanford, California 94305 THE LATERAL giant fiber is the decision fiber and fast flexor motoneurons are located in for a behavioral response in crayfish in- the dorsal neuropil of each abdominal gan- volving rapid tail flexion in response to glion. Dye injections and anatomical recon- abdominal tactile stimulation (27). Each structions of ‘identified motoneurons reveal lateral giant impulse is followed by a rapid that only those motoneurons which have tail flexion or tail flip, and when the giant dentritic branches in contact with a giant fiber does not fire in response to such stim- fiber are activated by that giant fiber (12, uli, the movement does not occur. 21). All of tl ie fast flexor motoneurons are The afferent limb of the reflex exciting excited by the ipsilateral giant; all but F4, the lateral giant has been described (28), F5 and F7 are also excited by the contra- and the habituation of the behavior has latkral giant (21 a). been explained in terms of the properties The excitation of these motoneurons, of this part of the neural circuit (29). seen as depolarizing potentials in their The properties of the neuromuscular somata, does not always reach threshold for junctions between the fast flexor motoneu- generating a spike which propagates out the rons and the phasic flexor muscles have also axon to the periphery (14). Indeed, only t,he been described (13). -

Plp-Positive Progenitor Cells Give Rise to Bergmann Glia in the Cerebellum
Citation: Cell Death and Disease (2013) 4, e546; doi:10.1038/cddis.2013.74 OPEN & 2013 Macmillan Publishers Limited All rights reserved 2041-4889/13 www.nature.com/cddis Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum S-H Chung1, F Guo2, P Jiang1, DE Pleasure2,3 and W Deng*,1,3,4 NG2 (nerve/glial antigen2)-expressing cells represent the largest population of postnatal progenitors in the central nervous system and have been classified as oligodendroglial progenitor cells, but the fate and function of these cells remain incompletely characterized. Previous studies have focused on characterizing these progenitors in the postnatal and adult subventricular zone and on analyzing the cellular and physiological properties of these cells in white and gray matter regions in the forebrain. In the present study, we examine the types of neural progeny generated by NG2 progenitors in the cerebellum by employing genetic fate mapping techniques using inducible Cre–Lox systems in vivo with two different mouse lines, the Plp-Cre-ERT2/Rosa26-EYFP and Olig2-Cre-ERT2/Rosa26-EYFP double-transgenic mice. Our data indicate that Olig2/Plp-positive NG2 cells display multipotential properties, primarily give rise to oligodendroglia but, surprisingly, also generate Bergmann glia, which are specialized glial cells in the cerebellum. The NG2 þ cells also give rise to astrocytes, but not neurons. In addition, we show that glutamate signaling is involved in distinct NG2 þ cell-fate/differentiation pathways and plays a role in the normal development of Bergmann glia. We also show an increase of cerebellar oligodendroglial lineage cells in response to hypoxic–ischemic injury, but the ability of NG2 þ cells to give rise to Bergmann glia and astrocytes remains unchanged. -

Neuregulin 1–Erbb2 Signaling Is Required for the Establishment of Radial Glia and Their Transformation Into Astrocytes in Cerebral Cortex
Neuregulin 1–erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex Ralf S. Schmid*, Barbara McGrath*, Bridget E. Berechid†, Becky Boyles*, Mark Marchionni‡, Nenad Sˇ estan†, and Eva S. Anton*§ *University of North Carolina Neuroscience Center and Department of Cell and Molecular Physiology, University of North Carolina School of Medicine, Chapel Hill, NC 27599; †Department of Neurobiology, Yale University School of Medicine, New Haven, CT 06510; and ‡CeNes Pharamceuticals, Inc., Norwood, MA 02062 Communicated by Pasko Rakic, Yale University School of Medicine, New Haven, CT, January 27, 2003 (received for review December 12, 2002) Radial glial cells and astrocytes function to support the construction mine whether NRG-1-mediated signaling is involved in radial and maintenance, respectively, of the cerebral cortex. However, the glial cell development and differentiation in the cerebral cortex. mechanisms that determine how radial glial cells are established, We show that NRG-1 signaling, involving erbB2, may act in maintained, and transformed into astrocytes in the cerebral cortex are concert with Notch signaling to exert a critical influence in the not well understood. Here, we show that neuregulin-1 (NRG-1) exerts establishment, maintenance, and appropriate transformation of a critical role in the establishment of radial glial cells. Radial glial cell radial glial cells in cerebral cortex. generation is significantly impaired in NRG mutants, and this defect can be rescued by exogenous NRG-1. Down-regulation of expression Materials and Methods and activity of erbB2, a member of the NRG-1 receptor complex, leads Clonal Analysis to Study NRG’s Role in the Initial Establishment of to the transformation of radial glial cells into astrocytes. -

Polyribosomes Associated with Synaptic Specializations on Axon Initial Segments: Localization of Protein-Synthetic Machinery at Inhibitory Synapses
The Journal of Neuroscience October 1986, 6(10): 3079-3085 Polyribosomes Associated with Synaptic Specializations on Axon Initial Segments: Localization of Protein-Synthetic Machinery at Inhibitory Synapses Oswald Steward* and Charles E. Ribak-f *Departments of Neurosurgery and Physiology, University of Virginia School of Medicine, Charlottesville, Virginia 22908, and TDepartment of Anatomy and Neurobiology, University of California at Irvine, Irvine, California 92717 Previous studies have revealed a selective association between report that showed some axonal ribosomes grouped at points polyribosomes and axospinous synapses in a variety of brain of branching and beneath synaptic contacts (Jones and Powell, regions. The present study evaluates whether polyribosomes are 1969). Therefore, they were usually considered, only in passing, also associated with the symmetrical and presumably inhibitory as exceptions to the rule. Thus, it has come to be accepted as a synaptic connections found on the initial segment of axons of central tenet that essentially all of the protein constituents of some neurons in the CNS. The initial segments of pyramidal the neuron, including those required for synaptic specializations, neurons in the sensorimotor cortex of the monkey and of granule were synthesized in the cell body, and somehow selectively cells in the hippocampus of the rat were examined. The initial transported to the sites at which they would be assembled (for segments of these cell types are contacted by GABAergic ter- a review, see Grafstein and Forman, 1980). minals that form symmetrical synaptic connections. In the pres- Over the past few years, a series of observations has given ent study, these initial segments were found to contain polyri- rise to an hypothesis that challenges the prevailing view that bosomes that tended to be selectively localized beneath the virtually all neuronal proteins are produced in the cell body and synaptic specializations. -

Neuron Morphology Influences Axon Initial Segment Plasticity
Dartmouth College Dartmouth Digital Commons Dartmouth Scholarship Faculty Work 1-2016 Neuron Morphology Influences Axon Initial Segment Plasticity Allan T. Gulledge Dartmouth College, [email protected] Jaime J. Bravo Dartmouth College Follow this and additional works at: https://digitalcommons.dartmouth.edu/facoa Part of the Computational Neuroscience Commons, Molecular and Cellular Neuroscience Commons, and the Other Neuroscience and Neurobiology Commons Dartmouth Digital Commons Citation Gulledge, A.T. and Bravo, J.J. (2016) Neuron morphology influences axon initial segment plasticity, eNeuro doi:10.1523/ENEURO.0085-15.2016. This Article is brought to you for free and open access by the Faculty Work at Dartmouth Digital Commons. It has been accepted for inclusion in Dartmouth Scholarship by an authorized administrator of Dartmouth Digital Commons. For more information, please contact [email protected]. New Research Neuronal Excitability Neuron Morphology Influences Axon Initial Segment Plasticity1,2,3 Allan T. Gulledge1 and Jaime J. Bravo2 DOI:http://dx.doi.org/10.1523/ENEURO.0085-15.2016 1Department of Physiology and Neurobiology, Geisel School of Medicine at Dartmouth, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire 03756, and 2Thayer School of Engineering at Dartmouth, Hanover, New Hampshire 03755 Visual Abstract In most vertebrate neurons, action potentials are initiated in the axon initial segment (AIS), a specialized region of the axon containing a high density of voltage-gated sodium and potassium channels. It has recently been proposed that neurons use plasticity of AIS length and/or location to regulate their intrinsic excitability. Here we quantify the impact of neuron morphology on AIS plasticity using computational models of simplified and realistic somatodendritic morphologies. -

Satellite Glial Cell Communication in Dorsal Root Ganglia
Neuronal somatic ATP release triggers neuron– satellite glial cell communication in dorsal root ganglia X. Zhang, Y. Chen, C. Wang, and L.-Y. M. Huang* Department of Neuroscience and Cell Biology, University of Texas Medical Branch, Galveston, TX 77555-1069 Edited by Charles F. Stevens, The Salk Institute for Biological Studies, La Jolla, CA, and approved April 25, 2007 (received for review December 14, 2006) It has been generally assumed that the cell body (soma) of a release of ATP from the somata of DRG neurons. Here, we show neuron, which contains the nucleus, is mainly responsible for that electric stimulation elicits robust vesicular release of ATP from synthesis of macromolecules and has a limited role in cell-to-cell neuronal somata and thus triggers bidirectional communication communication. Using sniffer patch recordings, we show here that between neurons and satellite cells. electrical stimulation of dorsal root ganglion (DRG) neurons elicits robust vesicular ATP release from their somata. The rate of release Results events increases with the frequency of nerve stimulation; external We asked first whether vesicular release of ATP occurs in the -Ca2؉ entry is required for the release. FM1–43 photoconversion somata of DRG neurons. P2X2-EGFP receptors were overex analysis further reveals that small clear vesicles participate in pressed in HEK cells and used as the biosensor (i.e., sniffer patch exocytosis. In addition, the released ATP activates P2X7 receptors method) (20). P2X2 receptors were chosen because they were easily in satellite cells that enwrap each DRG neuron and triggers the expressed at a high level (Ϸ200 pA/pF) in HEK cells and the communication between neuronal somata and glial cells. -

Was Not Reached, However, Even After Six to Sevenhours. A
PROTEIN SYNTHESIS IN THE ISOLATED GIANT AXON OF THE SQUID* BY A. GIUDITTA,t W.-D. DETTBARN,t AND MIROSLAv BRZIN§ MARINE BIOLOGICAL LABORATORY, WOODS HOLE, MASSACHUSETTS Communicated by David Nachmansohn, February 2, 1968 The work of Weiss and his associates,1-3 and more recently of a number of other investigators,4- has established the occurrence of a flux of materials from the soma of neurons toward the peripheral regions of the axon. It has been postulated that this mechanism would account for the origin of most of the axonal protein, although the time required to cover the distance which separates some axonal tips from their cell bodies would impose severe delays.4 On the other hand, a number of observations7-9 have indicated the occurrence of local mechanisms of synthesis in peripheral axons, as suggested by the kinetics of appearance of individual proteins after axonal transection. In this paper we report the incorporation of radioactive amino acids into the protein fraction of the axoplasm and of the axonal envelope obtained from giant axons of the squid. These axons are isolated essentially free from small fibers and connective tissue, and pure samples of axoplasm may be obtained by extru- sion of the axon. Incorporation of amino acids into axonal protein has recently been reported using systems from mammals'0 and fish."I Materials and Methods.-Giant axons of Loligo pealii were dissected and freed from small fibers: they were tied at both ends. Incubations were carried out at 18-20° in sea water previously filtered through Millipore which contained 5 mM Tris pH 7.8 and 10 Muc/ml of a mixture of 15 C'4-labeled amino acids (New England Nuclear Co., Boston, Mass.). -

Microglia Monitor and Protect Neuronal Function Via Specialized Somatic Purinergic Junctions
bioRxiv preprint doi: https://doi.org/10.1101/606079; this version posted April 13, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Microglia monitor and protect neuronal function via specialized somatic purinergic junctions Short title: Microglia control neurons at somatic junctions One-sentence summary: Neuronal cell bodies possess specialized, pre-formed sites, through which microglia monitor their status and exert neuroprotection. Csaba Cserép1,11, Balázs Pósfai1,10,11, Barbara Orsolits1, Gábor Molnár2, Steffanie Heindl3, Nikolett Lénárt1, Rebeka Fekete1,10, Zsófia I. László4,10, Zsolt Lele4, Anett D. Schwarcz1, Katinka Ujvári1, László Csiba5, Tibor Hortobágyi6, Zsófia Maglóczky7, Bernadett Martinecz1, Gábor Szabó8, Ferenc Erdélyi8, Róbert Szipőcs9, Benno Gesierich3, Marco Duering3, István Katona4, Arthur Liesz3, Gábor Tamás2, Ádám Dénes1,12,* 1 “Momentum” Laboratory of Neuroimmunology, Institute of Experimental Medicine, Hungarian Academy of Sciences, Hungary; 2 MTA-SZTE Research Group for Cortical Microcircuits, Department of Physiology, Anatomy and Neuroscience, University of Szeged, Hungary; 3 Institute for Stroke and Dementia Research, Ludwig-Maximilians-University, Munich, Germany; 4 “Momentum” Laboratory of Molecular Neurobiology, Institute of Experimental Medicine, Hungarian Academy of Sciences, Hungary; 5 MTA-DE Cerebrovascular -

Tecnicas Microscopicas
CAP 1: TÉCNICAS MICROSCÓPICAS TÉCNICAS MICROSCÓPICAS 11 Lic. Carlos R. Neira Montoya Lic. Eduardo Sedano Gelvet Lic. María Elena Vilcarromero V. El estudio de los tejidos tal como los observamos hoy en día no sería posible sin la ayuda de la histotecnología; esta disciplina se encarga del estudio de los métodos técnicas y procedimientos que permiten la transformación de un órgano en una película lo suficientemente transparente y contrastada que nos permite su observación a través del microscopio (Fig. 1-1). Para que esto ocurra se tiene que seguir una serie de pasos. Cada uno de estos pasos permite la observación de las características morfológicas del tejido que nos indica la normalidad o la alteración patológica; sin embargo en estudios mucho más minuciosos, estos pasos se harán en función de las estructuras o sustancias que se deseen investigar en la muestra correspondiente. Figura 1-1. Un órgano es transformado en una película transparente. - Pág. 5 - CAP 1: TÉCNICAS MICROSCÓPICAS Los pasos de las técnicas microscópicas para obtener un preparado histológico permanente (láminas) son: 1. Toma de la muestra. 2. Fijación. 3. Inclusión. 4. Microtomía. 5. Coloración. 1. TOMA DE LA MUESTRA Es el momento que se selecciona el órgano o tejido a estudiar. De tres fuentes puede provenir el material humano: las necropsias, las biopsias y las piezas operadas. De éstas, sólo la primera puede darnos material normal; las dos últimas habitualmente proporcionarán tejidos para estudio histopatológico. - Necropsias: son las piezas que se obtienen de un cadáver. Para histología normal es necesario que se trate de un cadáver fresco y que no haya sido atacado por ninguna lesión, por lo menos el órgano que se quiere estudiar. -

Imaging and Quantifying Ganglion Cells and Other Transparent Neurons in the Living Human Retina
Imaging and quantifying ganglion cells and other transparent neurons in the living human retina Zhuolin Liua,1, Kazuhiro Kurokawaa, Furu Zhanga, John J. Leeb, and Donald T. Millera aSchool of Optometry, Indiana University, Bloomington, IN 47405; and bPurdue School of Engineering and Technology, Indiana University–Purdue University Indianapolis, Indianapolis, IN 46202 Edited by David R. Williams, University of Rochester, Rochester, NY, and approved October 18, 2017 (received for review June 30, 2017) Ganglion cells (GCs) are fundamental to retinal neural circuitry, apoptotic GCs tagged with an intravenously administered fluores- processing photoreceptor signals for transmission to the brain via cent marker (14), thus providing direct monitoring of GC loss. The their axons. However, much remains unknown about their role in second incorporated adaptive optics (AO)—which corrects ocular vision and their vulnerability to disease leading to blindness. A aberrations—into SLO sensitive to multiply-scattered light (12). major bottleneck has been our inability to observe GCs and their This clever combination permitted imaging of a monolayer of GC degeneration in the living human eye. Despite two decades of layer (GCL) somas in areas with little or no overlying nerve fiber development of optical technologies to image cells in the living layer (NFL) (see figure 5, human result of Rossi et al.; ref. 12). By human retina, GCs remain elusive due to their high optical trans- contrast, our approach uses singly scattered light and produces lucency. Failure of conventional imaging—using predominately sin- images of unprecedented clarity of translucent retinal tissue. This gly scattered light—to reveal GCs has led to a focus on multiply- permits morphometry of GCL somas across the living human ret- scattered, fluorescence, two-photon, and phase imaging techniques ina. -

11 Introduction to the Nervous System and Nervous Tissue
11 Introduction to the Nervous System and Nervous Tissue ou can’t turn on the television or radio, much less go online, without seeing some- 11.1 Overview of the Nervous thing to remind you of the nervous system. From advertisements for medications System 381 Yto treat depression and other psychiatric conditions to stories about celebrities and 11.2 Nervous Tissue 384 their battles with illegal drugs, information about the nervous system is everywhere in 11.3 Electrophysiology our popular culture. And there is good reason for this—the nervous system controls our of Neurons 393 perception and experience of the world. In addition, it directs voluntary movement, and 11.4 Neuronal Synapses 406 is the seat of our consciousness, personality, and learning and memory. Along with the 11.5 Neurotransmitters 413 endocrine system, the nervous system regulates many aspects of homeostasis, including 11.6 Functional Groups respiratory rate, blood pressure, body temperature, the sleep/wake cycle, and blood pH. of Neurons 417 In this chapter we introduce the multitasking nervous system and its basic functions and divisions. We then examine the structure and physiology of the main tissue of the nervous system: nervous tissue. As you read, notice that many of the same principles you discovered in the muscle tissue chapter (see Chapter 10) apply here as well. MODULE 11.1 Overview of the Nervous System Learning Outcomes 1. Describe the major functions of the nervous system. 2. Describe the structures and basic functions of each organ of the central and peripheral nervous systems. 3. Explain the major differences between the two functional divisions of the peripheral nervous system.