Neuregulin 1–Erbb2 Signaling Is Required for the Establishment of Radial Glia and Their Transformation Into Astrocytes in Cerebral Cortex
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
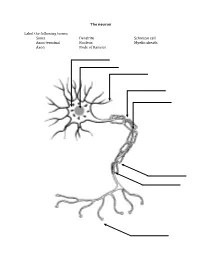
The Neuron Label the Following Terms: Soma Axon Terminal Axon Dendrite
The neuron Label the following terms: Soma Dendrite Schwaan cell Axon terminal Nucleus Myelin sheath Axon Node of Ranvier Neuron Vocabulary You must know the definitions of these terms 1. Synaptic Cleft 2. Neuron 3. Impulse 4. Sensory Neuron 5. Motor Neuron 6. Interneuron 7. Body (Soma) 8. Dendrite 9. Axon 10. Action Potential 11. Myelin Sheath (Myelin) 12. Afferent Neuron 13. Threshold 14. Neurotransmitter 15. Efferent Neurons 16. Axon Terminal 17. Stimulus 18. Refractory Period 19. Schwann 20. Nodes of Ranvier 21. Acetylcholine STEPS IN THE ACTION POTENTIAL 1. The presynaptic neuron sends neurotransmitters to postsynaptic neuron. 2. Neurotransmitters bind to receptors on the postsynaptic cell. - This action will either excite or inhibit the postsynaptic cell. - The soma becomes more positive. 3. The positive charge reaches the axon hillock. - Once the threshold of excitation is reached the neuron will fire an action potential. 4. Na+ channels open and Na+ is forced into the cell by the concentration gradient and the electrical gradient. - The neuron begins to depolarize. 5. The K+ channels open and K+ is forced out of the cell by the concentration gradient and the electrical gradient. - The neuron is depolarized. 6. The Na+ channels close at the peak of the action potential. - The neuron starts to repolarize. 7. The K+ channels close, but they close slowly and K+ leaks out. 8. The terminal buttons release neurotransmitter to the postsynaptic neuron. 9. The resting potential is overshot and the neuron falls to a -90mV (hyperpolarizes). - The neuron continues to repolarize. 10. The neuron returns to resting potential. The Synapse Label the following terms: Pre-synaptic membrane Neurotransmitters Post-synaptic membrane Synaptic cleft Vesicle Post-synaptic receptors . -

Crayfish Escape Behavior and Central Synapses
Crayfish Escape Behavior and Central Synapses. III. Electrical Junctions and Dendrite Spikes in Fast Flexor Motoneurons ROBERT S. ZUCKER Department of Biological Sciences and Program in the Neurological Sciences, Stanford University, Stanford, California 94305 THE LATERAL giant fiber is the decision fiber and fast flexor motoneurons are located in for a behavioral response in crayfish in- the dorsal neuropil of each abdominal gan- volving rapid tail flexion in response to glion. Dye injections and anatomical recon- abdominal tactile stimulation (27). Each structions of ‘identified motoneurons reveal lateral giant impulse is followed by a rapid that only those motoneurons which have tail flexion or tail flip, and when the giant dentritic branches in contact with a giant fiber does not fire in response to such stim- fiber are activated by that giant fiber (12, uli, the movement does not occur. 21). All of tl ie fast flexor motoneurons are The afferent limb of the reflex exciting excited by the ipsilateral giant; all but F4, the lateral giant has been described (28), F5 and F7 are also excited by the contra- and the habituation of the behavior has latkral giant (21 a). been explained in terms of the properties The excitation of these motoneurons, of this part of the neural circuit (29). seen as depolarizing potentials in their The properties of the neuromuscular somata, does not always reach threshold for junctions between the fast flexor motoneu- generating a spike which propagates out the rons and the phasic flexor muscles have also axon to the periphery (14). Indeed, only t,he been described (13). -

Plp-Positive Progenitor Cells Give Rise to Bergmann Glia in the Cerebellum
Citation: Cell Death and Disease (2013) 4, e546; doi:10.1038/cddis.2013.74 OPEN & 2013 Macmillan Publishers Limited All rights reserved 2041-4889/13 www.nature.com/cddis Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum S-H Chung1, F Guo2, P Jiang1, DE Pleasure2,3 and W Deng*,1,3,4 NG2 (nerve/glial antigen2)-expressing cells represent the largest population of postnatal progenitors in the central nervous system and have been classified as oligodendroglial progenitor cells, but the fate and function of these cells remain incompletely characterized. Previous studies have focused on characterizing these progenitors in the postnatal and adult subventricular zone and on analyzing the cellular and physiological properties of these cells in white and gray matter regions in the forebrain. In the present study, we examine the types of neural progeny generated by NG2 progenitors in the cerebellum by employing genetic fate mapping techniques using inducible Cre–Lox systems in vivo with two different mouse lines, the Plp-Cre-ERT2/Rosa26-EYFP and Olig2-Cre-ERT2/Rosa26-EYFP double-transgenic mice. Our data indicate that Olig2/Plp-positive NG2 cells display multipotential properties, primarily give rise to oligodendroglia but, surprisingly, also generate Bergmann glia, which are specialized glial cells in the cerebellum. The NG2 þ cells also give rise to astrocytes, but not neurons. In addition, we show that glutamate signaling is involved in distinct NG2 þ cell-fate/differentiation pathways and plays a role in the normal development of Bergmann glia. We also show an increase of cerebellar oligodendroglial lineage cells in response to hypoxic–ischemic injury, but the ability of NG2 þ cells to give rise to Bergmann glia and astrocytes remains unchanged. -

Oligodendrocytes in Development, Myelin Generation and Beyond
cells Review Oligodendrocytes in Development, Myelin Generation and Beyond Sarah Kuhn y, Laura Gritti y, Daniel Crooks and Yvonne Dombrowski * Wellcome-Wolfson Institute for Experimental Medicine, Queen’s University Belfast, Belfast BT9 7BL, UK; [email protected] (S.K.); [email protected] (L.G.); [email protected] (D.C.) * Correspondence: [email protected]; Tel.: +0044-28-9097-6127 These authors contributed equally. y Received: 15 October 2019; Accepted: 7 November 2019; Published: 12 November 2019 Abstract: Oligodendrocytes are the myelinating cells of the central nervous system (CNS) that are generated from oligodendrocyte progenitor cells (OPC). OPC are distributed throughout the CNS and represent a pool of migratory and proliferative adult progenitor cells that can differentiate into oligodendrocytes. The central function of oligodendrocytes is to generate myelin, which is an extended membrane from the cell that wraps tightly around axons. Due to this energy consuming process and the associated high metabolic turnover oligodendrocytes are vulnerable to cytotoxic and excitotoxic factors. Oligodendrocyte pathology is therefore evident in a range of disorders including multiple sclerosis, schizophrenia and Alzheimer’s disease. Deceased oligodendrocytes can be replenished from the adult OPC pool and lost myelin can be regenerated during remyelination, which can prevent axonal degeneration and can restore function. Cell population studies have recently identified novel immunomodulatory functions of oligodendrocytes, the implications of which, e.g., for diseases with primary oligodendrocyte pathology, are not yet clear. Here, we review the journey of oligodendrocytes from the embryonic stage to their role in homeostasis and their fate in disease. We will also discuss the most common models used to study oligodendrocytes and describe newly discovered functions of oligodendrocytes. -

Pre-Oligodendrocytes from Adult Human CNS
The Journal of Neuroscience, April 1992, 12(4): 1538-l 547 Pre-Oligodendrocytes from Adult Human CNS Regina C. Armstrong,lJ Henry H. Dorn, l,b Conrad V. Kufta,* Emily Friedman,3 and Monique E. Dubois-Dalcq’ ‘Laboratory of Viral and Molecular Pathogenesis, and %urgical Neurology Branch, National Institute of Neurological Disorders and Stroke, Bethesda, Maryland 20892 and 3Department of Neurosurgery, University of Pennsylvania, Philadelphia, Pennsylvania 19104-3246 CNS remyelination and functional recovery often occur after Rapid and efficient neurotransmission is dependent upon the experimental demyelination in adult rodents. This has been electrical insulating capacity of the myelin sheath around axons attributed to the ability of mature oligodendrocytes and/or (reviewed in Ritchie, 1984a,b). Nerve conduction is impaired their precursor cells to divide and regenerate in response after loss of the myelin sheath and results in severe neurological to signals in demyelinating lesions. To determine whether dysfunction in human demyelinating diseases such as multiple oligodendrocyte precursor cells exist in the adult human sclerosis (MS). Remyelination can occur in the CNS of MS CNS, we have cultured white matter from patients under- patients but appears to be limited (Perier and Gregoire, 1965; going partial temporal lobe resection for intractable epilep- Prineas et al., 1984). Studies of acute MS cases have revealed sy. These cultures contained a population of process-bear- that recent demyelinating lesions can exhibit remyelination that ing cells that expressed antigens recognized by the 04 appears to correlate with the generation of new oligodendrocytes monoclonal antibody, but these cells did not express galac- (Prineas et al., 1984; Raine et al., 1988). -

Regulation of Adult Neurogenesis in Mammalian Brain
International Journal of Molecular Sciences Review Regulation of Adult Neurogenesis in Mammalian Brain 1,2, 3, 3,4 Maria Victoria Niklison-Chirou y, Massimiliano Agostini y, Ivano Amelio and Gerry Melino 3,* 1 Centre for Therapeutic Innovation (CTI-Bath), Department of Pharmacy & Pharmacology, University of Bath, Bath BA2 7AY, UK; [email protected] 2 Blizard Institute of Cell and Molecular Science, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London E1 2AT, UK 3 Department of Experimental Medicine, TOR, University of Rome “Tor Vergata”, 00133 Rome, Italy; [email protected] (M.A.); [email protected] (I.A.) 4 School of Life Sciences, University of Nottingham, Nottingham NG7 2HU, UK * Correspondence: [email protected] These authors contributed equally to this work. y Received: 18 May 2020; Accepted: 7 July 2020; Published: 9 July 2020 Abstract: Adult neurogenesis is a multistage process by which neurons are generated and integrated into existing neuronal circuits. In the adult brain, neurogenesis is mainly localized in two specialized niches, the subgranular zone (SGZ) of the dentate gyrus and the subventricular zone (SVZ) adjacent to the lateral ventricles. Neurogenesis plays a fundamental role in postnatal brain, where it is required for neuronal plasticity. Moreover, perturbation of adult neurogenesis contributes to several human diseases, including cognitive impairment and neurodegenerative diseases. The interplay between extrinsic and intrinsic factors is fundamental in regulating neurogenesis. Over the past decades, several studies on intrinsic pathways, including transcription factors, have highlighted their fundamental role in regulating every stage of neurogenesis. However, it is likely that transcriptional regulation is part of a more sophisticated regulatory network, which includes epigenetic modifications, non-coding RNAs and metabolic pathways. -

The Interplay Between Neurons and Glia in Synapse Development And
Available online at www.sciencedirect.com ScienceDirect The interplay between neurons and glia in synapse development and plasticity Jeff A Stogsdill and Cagla Eroglu In the brain, the formation of complex neuronal networks and regulate distinct aspects of synaptic development and amenable to experience-dependent remodeling is complicated circuit connectivity. by the diversity of neurons and synapse types. The establishment of a functional brain depends not only on The intricate communication between neurons and glia neurons, but also non-neuronal glial cells. Glia are in and their cooperative roles in synapse formation are now continuous bi-directional communication with neurons to direct coming to light due in large part to advances in genetic the formation and refinement of synaptic connectivity. This and imaging tools. This article will examine the progress article reviews important findings, which uncovered cellular made in our understanding of the role of mammalian and molecular aspects of the neuron–glia cross-talk that perisynaptic glia (astrocytes and microglia) in synapse govern the formation and remodeling of synapses and circuits. development, maturation, and plasticity since the previ- In vivo evidence demonstrating the critical interplay between ous Current Opinion article [1]. An integration of past and neurons and glia will be the major focus. Additional attention new findings of glial control of synapse development and will be given to how aberrant communication between neurons plasticity is tabulated in Box 1. and glia may contribute to neural pathologies. Address Glia control the formation of synaptic circuits Department of Cell Biology, Duke University Medical Center, Durham, In the CNS, glial cells are in tight association with NC 27710, USA synapses in all brain regions [2]. -

A System for Studying Mechanisms of Neuromuscular Junction Development and Maintenance Valérie Vilmont1,‡, Bruno Cadot1, Gilles Ouanounou2 and Edgar R
© 2016. Published by The Company of Biologists Ltd | Development (2016) 143, 2464-2477 doi:10.1242/dev.130278 TECHNIQUES AND RESOURCES RESEARCH ARTICLE A system for studying mechanisms of neuromuscular junction development and maintenance Valérie Vilmont1,‡, Bruno Cadot1, Gilles Ouanounou2 and Edgar R. Gomes1,3,*,‡ ABSTRACT different animal models and cell lines (Chen et al., 2014; Corti et al., The neuromuscular junction (NMJ), a cellular synapse between a 2012; Lenzi et al., 2015) with the hope of recapitulating some motor neuron and a skeletal muscle fiber, enables the translation of features of neuromuscular diseases and understanding the triggers chemical cues into physical activity. The development of this special of one of their common hallmarks: the disruption of the structure has been subject to numerous investigations, but its neuromuscular junction (NMJ). The NMJ is one of the most complexity renders in vivo studies particularly difficult to perform. studied synapses. It is formed of three key elements: the lower motor In vitro modeling of the neuromuscular junction represents a powerful neuron (the pre-synaptic compartment), the skeletal muscle (the tool to delineate fully the fine tuning of events that lead to subcellular post-synaptic compartment) and the Schwann cell (Sanes and specialization at the pre-synaptic and post-synaptic sites. Here, we Lichtman, 1999). The NMJ is formed in a step-wise manner describe a novel heterologous co-culture in vitro method using rat following a series of cues involving these three cellular components spinal cord explants with dorsal root ganglia and murine primary and its role is basically to ensure the skeletal muscle functionality. -

Differential Timing and Coordination of Neurogenesis and Astrogenesis
brain sciences Article Differential Timing and Coordination of Neurogenesis and Astrogenesis in Developing Mouse Hippocampal Subregions Allison M. Bond 1, Daniel A. Berg 1, Stephanie Lee 1, Alan S. Garcia-Epelboim 1, Vijay S. Adusumilli 1, Guo-li Ming 1,2,3,4 and Hongjun Song 1,2,3,5,* 1 Department of Neuroscience and Mahoney Institute for Neurosciences, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; [email protected] (A.M.B.); [email protected] (D.A.B.); [email protected] (S.L.); [email protected] (A.S.G.-E.); [email protected] (V.S.A.); [email protected] (G.-l.M.) 2 Department of Cell and Developmental Biology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA 3 Institute for Regenerative Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA 4 Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA 5 The Epigenetics Institute, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA * Correspondence: [email protected] Received: 19 October 2020; Accepted: 24 November 2020; Published: 26 November 2020 Abstract: Neocortical development has been extensively studied and therefore is the basis of our understanding of mammalian brain development. One fundamental principle of neocortical development is that neurogenesis and gliogenesis are temporally segregated processes. However, it is unclear how neurogenesis and gliogenesis are coordinated in non-neocortical regions of the cerebral cortex, such as the hippocampus, also known as the archicortex. Here, we show that the timing of neurogenesis and astrogenesis in the Cornu Ammonis (CA) 1 and CA3 regions of mouse hippocampus mirrors that of the neocortex; neurogenesis occurs embryonically, followed by astrogenesis during early postnatal development. -

NEUROGENESIS in the ADULT BRAIN: New Strategies for Central Nervous System Diseases
7 Jan 2004 14:25 AR AR204-PA44-17.tex AR204-PA44-17.sgm LaTeX2e(2002/01/18) P1: GCE 10.1146/annurev.pharmtox.44.101802.121631 Annu. Rev. Pharmacol. Toxicol. 2004. 44:399–421 doi: 10.1146/annurev.pharmtox.44.101802.121631 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on August 28, 2003 NEUROGENESIS IN THE ADULT BRAIN: New Strategies for Central Nervous System Diseases ,1 ,2 D. Chichung Lie, Hongjun Song, Sophia A. Colamarino,1 Guo-li Ming,2 and Fred H. Gage1 1Laboratory of Genetics, The Salk Institute, La Jolla, California 92037; email: [email protected], [email protected], [email protected] 2Institute for Cell Engineering, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21287; email: [email protected], [email protected] Key Words adult neural stem cells, regeneration, recruitment, cell replacement, therapy ■ Abstract New cells are continuously generated from immature proliferating cells throughout adulthood in many organs, thereby contributing to the integrity of the tissue under physiological conditions and to repair following injury. In contrast, repair mechanisms in the adult central nervous system (CNS) have long been thought to be very limited. However, recent findings have clearly demonstrated that in restricted areas of the mammalian brain, new functional neurons are constantly generated from neural stem cells throughout life. Moreover, stem cells with the potential to give rise to new neurons reside in many different regions of the adult CNS. These findings raise the possibility that endogenous neural stem cells can be mobilized to replace dying neurons in neurodegenerative diseases. -

Endogenous Radial Glial Cells Support Regenerating Axons After Spinal
Cellular, molecular, and developmental neuroscience 871 Endogenous radial glial cells support regenerating axons after spinal cord transection Hiroshi Nomuraa, Howard Kimb, Andrea Mothea, Tasneem Zahirb, Iris Kulbatskia, Cindi M. Morsheadc, Molly S. Shoichetb and Charles H. Tatora During the development of central nervous system, radial NeuroReport 21:871–876 c 2010 Wolters Kluwer Health | glial cells support target-specific neuronal migration. Lippincott Williams & Wilkins. We recently reported that after implantation of chitosan NeuroReport 2010, 21:871–876 channels with complete spinal cord transection, the tissue bridging the spinal cord stumps contained axons and radial Keywords: channel implantation, chitosan, radial glial cell, spinal cord injury, spinal cord transection glial cells. The purpose of this study was to clarify the role of the radial glial cells in the tissue bridges. Chitosan aToronto Western Research Institute, Toronto Western Hospital, bDepartment of Chemical Engineering and Applied Chemistry and cDepartment of Surgery and channels were implanted in rats with thoracic spinal Institute of Medical Sciences, University of Toronto, Terrence Donnelly Centre for cord transection. After 14 weeks, all animals had tissue Cellular and Biomolecular Research, Toronto, Ontario, Canada bridges in the channels that contained many radial glial Correspondence to Charles H. Tator, MD, PhD, Toronto Western Research cells in longitudinal arrangement, some of which were Institute, Toronto Western Hospital, Room 12-423, McLaughlin Wing, 399 Bathurst Street, Toronto, Ontario M5T 2S8, Canada in contact with axons in the bridges. We suggest that Tel: + 1 416 603 5889; fax: + 1 416 603 5298; e-mail:[email protected] radial glial cells can guide regenerating axons across Received 25 May 2010 accepted 25 June 2010 the bridge in the channel after spinal cord transection. -

Modeling Cortical Development and Microcephaly in Human Pluripotent Stem Cells Using Combinatorial Pathway Inhibition
Modeling cortical development and microcephaly in human pluripotent stem cells using combinatorial pathway inhibition Dissertation to obtain the academic degree Doctor rerum naturalium (Dr. rer. nat.) submitted to the Department of Biology, Chemistry and Pharmacy of Freie Universität Berlin by Naresh Mutukula 2019 The research work for this dissertation was performed from July 2014 to May 2019 under the supervision of Dr. Yechiel Elkabetz at the Max Planck Institute for Molecular Genetics in Berlin, Germany. The dissertation was submitted in May 2019 to the Department of Biology, Chemistry and Pharmacy of the Freie Universität Berlin, Germany. 1st Reviewer: Dr. Yechiel Elkabetz Max Planck Institute for Molecular Genetics, Berlin. 2nd Reviewer: Prof. Dr. Sigmar Stricker Freie Universität Berlin Date of disputation: 18th Nov, 2019 Acknowledgements First and foremost, I would like to express my deep gratitude to my supervisor Dr. Yechiel Elkabetz for giving me the opportunity to work in his lab and introducing me to the very exciting world of pluripotent and neural stem cell biology research. I am very grateful to him for his excellent supervision, constant support and immense patience he has shown in both good and bad times during all these years of my PhD. I would like to thank him for all the teachings and discussions including non- academics during the last few years. Secondly, I would like to thank Prof. Dr. Sigmar Stricker for accepting to be the second reviewer of my PhD dissertation. I am very thankful to my good friend Rotem Volkman from Tel Aviv University, who was there for me during the initial phase of my PhD.