Crayfish Escape Behavior and Central Synapses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
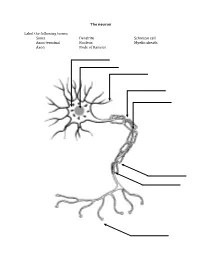
The Neuron Label the Following Terms: Soma Axon Terminal Axon Dendrite
The neuron Label the following terms: Soma Dendrite Schwaan cell Axon terminal Nucleus Myelin sheath Axon Node of Ranvier Neuron Vocabulary You must know the definitions of these terms 1. Synaptic Cleft 2. Neuron 3. Impulse 4. Sensory Neuron 5. Motor Neuron 6. Interneuron 7. Body (Soma) 8. Dendrite 9. Axon 10. Action Potential 11. Myelin Sheath (Myelin) 12. Afferent Neuron 13. Threshold 14. Neurotransmitter 15. Efferent Neurons 16. Axon Terminal 17. Stimulus 18. Refractory Period 19. Schwann 20. Nodes of Ranvier 21. Acetylcholine STEPS IN THE ACTION POTENTIAL 1. The presynaptic neuron sends neurotransmitters to postsynaptic neuron. 2. Neurotransmitters bind to receptors on the postsynaptic cell. - This action will either excite or inhibit the postsynaptic cell. - The soma becomes more positive. 3. The positive charge reaches the axon hillock. - Once the threshold of excitation is reached the neuron will fire an action potential. 4. Na+ channels open and Na+ is forced into the cell by the concentration gradient and the electrical gradient. - The neuron begins to depolarize. 5. The K+ channels open and K+ is forced out of the cell by the concentration gradient and the electrical gradient. - The neuron is depolarized. 6. The Na+ channels close at the peak of the action potential. - The neuron starts to repolarize. 7. The K+ channels close, but they close slowly and K+ leaks out. 8. The terminal buttons release neurotransmitter to the postsynaptic neuron. 9. The resting potential is overshot and the neuron falls to a -90mV (hyperpolarizes). - The neuron continues to repolarize. 10. The neuron returns to resting potential. The Synapse Label the following terms: Pre-synaptic membrane Neurotransmitters Post-synaptic membrane Synaptic cleft Vesicle Post-synaptic receptors . -

Long-Term Adult Human Brain Slice Cultures As a Model System to Study
TOOLS AND RESOURCES Long-term adult human brain slice cultures as a model system to study human CNS circuitry and disease Niklas Schwarz1, Betu¨ l Uysal1, Marc Welzer2,3, Jacqueline C Bahr1, Nikolas Layer1, Heidi Lo¨ ffler1, Kornelijus Stanaitis1, Harshad PA1, Yvonne G Weber1,4, Ulrike BS Hedrich1, Ju¨ rgen B Honegger4, Angelos Skodras2,3, Albert J Becker5, Thomas V Wuttke1,4†*, Henner Koch1†* 1Department of Neurology and Epileptology, Hertie-Institute for Clinical Brain Research, University of Tu¨ bingen, Tu¨ bingen, Germany; 2Department of Cellular Neurology, Hertie-Institute for Clinical Brain Research, University of Tu¨ bingen, Tu¨ bingen, Germany; 3German Center for Neurodegenerative Diseases (DZNE), Tu¨ bingen, Germany; 4Department of Neurosurgery, University of Tu¨ bingen, Tu¨ bingen, Germany; 5Department of Neuropathology, Section for Translational Epilepsy Research, University Bonn Medical Center, Bonn, Germany Abstract Most of our knowledge on human CNS circuitry and related disorders originates from model organisms. How well such data translate to the human CNS remains largely to be determined. Human brain slice cultures derived from neurosurgical resections may offer novel avenues to approach this translational gap. We now demonstrate robust preservation of the complex neuronal cytoarchitecture and electrophysiological properties of human pyramidal neurons *For correspondence: in long-term brain slice cultures. Further experiments delineate the optimal conditions for efficient [email protected] viral transduction of cultures, enabling ‘high throughput’ fluorescence-mediated 3D reconstruction tuebingen.de (TVW); of genetically targeted neurons at comparable quality to state-of-the-art biocytin fillings, and [email protected] demonstrate feasibility of long term live cell imaging of human cells in vitro. -

Plp-Positive Progenitor Cells Give Rise to Bergmann Glia in the Cerebellum
Citation: Cell Death and Disease (2013) 4, e546; doi:10.1038/cddis.2013.74 OPEN & 2013 Macmillan Publishers Limited All rights reserved 2041-4889/13 www.nature.com/cddis Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum S-H Chung1, F Guo2, P Jiang1, DE Pleasure2,3 and W Deng*,1,3,4 NG2 (nerve/glial antigen2)-expressing cells represent the largest population of postnatal progenitors in the central nervous system and have been classified as oligodendroglial progenitor cells, but the fate and function of these cells remain incompletely characterized. Previous studies have focused on characterizing these progenitors in the postnatal and adult subventricular zone and on analyzing the cellular and physiological properties of these cells in white and gray matter regions in the forebrain. In the present study, we examine the types of neural progeny generated by NG2 progenitors in the cerebellum by employing genetic fate mapping techniques using inducible Cre–Lox systems in vivo with two different mouse lines, the Plp-Cre-ERT2/Rosa26-EYFP and Olig2-Cre-ERT2/Rosa26-EYFP double-transgenic mice. Our data indicate that Olig2/Plp-positive NG2 cells display multipotential properties, primarily give rise to oligodendroglia but, surprisingly, also generate Bergmann glia, which are specialized glial cells in the cerebellum. The NG2 þ cells also give rise to astrocytes, but not neurons. In addition, we show that glutamate signaling is involved in distinct NG2 þ cell-fate/differentiation pathways and plays a role in the normal development of Bergmann glia. We also show an increase of cerebellar oligodendroglial lineage cells in response to hypoxic–ischemic injury, but the ability of NG2 þ cells to give rise to Bergmann glia and astrocytes remains unchanged. -

Neuregulin 1–Erbb2 Signaling Is Required for the Establishment of Radial Glia and Their Transformation Into Astrocytes in Cerebral Cortex
Neuregulin 1–erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex Ralf S. Schmid*, Barbara McGrath*, Bridget E. Berechid†, Becky Boyles*, Mark Marchionni‡, Nenad Sˇ estan†, and Eva S. Anton*§ *University of North Carolina Neuroscience Center and Department of Cell and Molecular Physiology, University of North Carolina School of Medicine, Chapel Hill, NC 27599; †Department of Neurobiology, Yale University School of Medicine, New Haven, CT 06510; and ‡CeNes Pharamceuticals, Inc., Norwood, MA 02062 Communicated by Pasko Rakic, Yale University School of Medicine, New Haven, CT, January 27, 2003 (received for review December 12, 2002) Radial glial cells and astrocytes function to support the construction mine whether NRG-1-mediated signaling is involved in radial and maintenance, respectively, of the cerebral cortex. However, the glial cell development and differentiation in the cerebral cortex. mechanisms that determine how radial glial cells are established, We show that NRG-1 signaling, involving erbB2, may act in maintained, and transformed into astrocytes in the cerebral cortex are concert with Notch signaling to exert a critical influence in the not well understood. Here, we show that neuregulin-1 (NRG-1) exerts establishment, maintenance, and appropriate transformation of a critical role in the establishment of radial glial cells. Radial glial cell radial glial cells in cerebral cortex. generation is significantly impaired in NRG mutants, and this defect can be rescued by exogenous NRG-1. Down-regulation of expression Materials and Methods and activity of erbB2, a member of the NRG-1 receptor complex, leads Clonal Analysis to Study NRG’s Role in the Initial Establishment of to the transformation of radial glial cells into astrocytes. -

Neuron Morphology Influences Axon Initial Segment Plasticity
Dartmouth College Dartmouth Digital Commons Dartmouth Scholarship Faculty Work 1-2016 Neuron Morphology Influences Axon Initial Segment Plasticity Allan T. Gulledge Dartmouth College, [email protected] Jaime J. Bravo Dartmouth College Follow this and additional works at: https://digitalcommons.dartmouth.edu/facoa Part of the Computational Neuroscience Commons, Molecular and Cellular Neuroscience Commons, and the Other Neuroscience and Neurobiology Commons Dartmouth Digital Commons Citation Gulledge, A.T. and Bravo, J.J. (2016) Neuron morphology influences axon initial segment plasticity, eNeuro doi:10.1523/ENEURO.0085-15.2016. This Article is brought to you for free and open access by the Faculty Work at Dartmouth Digital Commons. It has been accepted for inclusion in Dartmouth Scholarship by an authorized administrator of Dartmouth Digital Commons. For more information, please contact [email protected]. New Research Neuronal Excitability Neuron Morphology Influences Axon Initial Segment Plasticity1,2,3 Allan T. Gulledge1 and Jaime J. Bravo2 DOI:http://dx.doi.org/10.1523/ENEURO.0085-15.2016 1Department of Physiology and Neurobiology, Geisel School of Medicine at Dartmouth, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire 03756, and 2Thayer School of Engineering at Dartmouth, Hanover, New Hampshire 03755 Visual Abstract In most vertebrate neurons, action potentials are initiated in the axon initial segment (AIS), a specialized region of the axon containing a high density of voltage-gated sodium and potassium channels. It has recently been proposed that neurons use plasticity of AIS length and/or location to regulate their intrinsic excitability. Here we quantify the impact of neuron morphology on AIS plasticity using computational models of simplified and realistic somatodendritic morphologies. -

Satellite Glial Cell Communication in Dorsal Root Ganglia
Neuronal somatic ATP release triggers neuron– satellite glial cell communication in dorsal root ganglia X. Zhang, Y. Chen, C. Wang, and L.-Y. M. Huang* Department of Neuroscience and Cell Biology, University of Texas Medical Branch, Galveston, TX 77555-1069 Edited by Charles F. Stevens, The Salk Institute for Biological Studies, La Jolla, CA, and approved April 25, 2007 (received for review December 14, 2006) It has been generally assumed that the cell body (soma) of a release of ATP from the somata of DRG neurons. Here, we show neuron, which contains the nucleus, is mainly responsible for that electric stimulation elicits robust vesicular release of ATP from synthesis of macromolecules and has a limited role in cell-to-cell neuronal somata and thus triggers bidirectional communication communication. Using sniffer patch recordings, we show here that between neurons and satellite cells. electrical stimulation of dorsal root ganglion (DRG) neurons elicits robust vesicular ATP release from their somata. The rate of release Results events increases with the frequency of nerve stimulation; external We asked first whether vesicular release of ATP occurs in the -Ca2؉ entry is required for the release. FM1–43 photoconversion somata of DRG neurons. P2X2-EGFP receptors were overex analysis further reveals that small clear vesicles participate in pressed in HEK cells and used as the biosensor (i.e., sniffer patch exocytosis. In addition, the released ATP activates P2X7 receptors method) (20). P2X2 receptors were chosen because they were easily in satellite cells that enwrap each DRG neuron and triggers the expressed at a high level (Ϸ200 pA/pF) in HEK cells and the communication between neuronal somata and glial cells. -

The Narrowing of Dendrite Branches Across Nodes Follows a Well-Defined Scaling Law
The narrowing of dendrite branches across nodes follows a well-defined scaling law Maijia Liaoa, Xin Liangb, and Jonathon Howarda,1 aDepartment of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT 06520; and bTsinghua-Peking Joint Center for Life Sciences, School of Life Sciences, Tsinghua University, 100084 Beijing, China Edited by Yuh Nung Jan, Howard Hughes Medical Institute, University of California San Francisco, San Francisco, CA, and approved May 17, 2021 (received for review October 28, 2020) The systematic variation of diameters in branched networks has minimized. Da Vinci’s law has been reformulated as the “pipe tantalized biologists since the discovery of da Vinci’s rule for trees. model” for plants, in which a fixed cross-section of stems and Da Vinci’s rule can be formulated as a power law with exponent branches is required to support each unit amount of leaves (13). two: The square of the mother branch’s diameter is equal to the Experimental support for scaling laws, however, is scarce because sum of the squares of those of the daughters. Power laws, with of the difficulties of imaging entire branched networks and because different exponents, have been proposed for branching in circula- intrinsic anatomical variability may obscure precise laws (14). Thus, tory systems (Murray’s law with exponent 3) and in neurons (Rall’s it is an open question whether scaling laws such as Eq. 1 apply in law with exponent 3/2). The laws have been derived theoretically, biological systems. based on optimality arguments, but, for the most part, have not To quantitatively test diameter-scaling laws in neurons, it is been tested rigorously. -

Was Not Reached, However, Even After Six to Sevenhours. A
PROTEIN SYNTHESIS IN THE ISOLATED GIANT AXON OF THE SQUID* BY A. GIUDITTA,t W.-D. DETTBARN,t AND MIROSLAv BRZIN§ MARINE BIOLOGICAL LABORATORY, WOODS HOLE, MASSACHUSETTS Communicated by David Nachmansohn, February 2, 1968 The work of Weiss and his associates,1-3 and more recently of a number of other investigators,4- has established the occurrence of a flux of materials from the soma of neurons toward the peripheral regions of the axon. It has been postulated that this mechanism would account for the origin of most of the axonal protein, although the time required to cover the distance which separates some axonal tips from their cell bodies would impose severe delays.4 On the other hand, a number of observations7-9 have indicated the occurrence of local mechanisms of synthesis in peripheral axons, as suggested by the kinetics of appearance of individual proteins after axonal transection. In this paper we report the incorporation of radioactive amino acids into the protein fraction of the axoplasm and of the axonal envelope obtained from giant axons of the squid. These axons are isolated essentially free from small fibers and connective tissue, and pure samples of axoplasm may be obtained by extru- sion of the axon. Incorporation of amino acids into axonal protein has recently been reported using systems from mammals'0 and fish."I Materials and Methods.-Giant axons of Loligo pealii were dissected and freed from small fibers: they were tied at both ends. Incubations were carried out at 18-20° in sea water previously filtered through Millipore which contained 5 mM Tris pH 7.8 and 10 Muc/ml of a mixture of 15 C'4-labeled amino acids (New England Nuclear Co., Boston, Mass.). -

Microglia Monitor and Protect Neuronal Function Via Specialized Somatic Purinergic Junctions
bioRxiv preprint doi: https://doi.org/10.1101/606079; this version posted April 13, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Microglia monitor and protect neuronal function via specialized somatic purinergic junctions Short title: Microglia control neurons at somatic junctions One-sentence summary: Neuronal cell bodies possess specialized, pre-formed sites, through which microglia monitor their status and exert neuroprotection. Csaba Cserép1,11, Balázs Pósfai1,10,11, Barbara Orsolits1, Gábor Molnár2, Steffanie Heindl3, Nikolett Lénárt1, Rebeka Fekete1,10, Zsófia I. László4,10, Zsolt Lele4, Anett D. Schwarcz1, Katinka Ujvári1, László Csiba5, Tibor Hortobágyi6, Zsófia Maglóczky7, Bernadett Martinecz1, Gábor Szabó8, Ferenc Erdélyi8, Róbert Szipőcs9, Benno Gesierich3, Marco Duering3, István Katona4, Arthur Liesz3, Gábor Tamás2, Ádám Dénes1,12,* 1 “Momentum” Laboratory of Neuroimmunology, Institute of Experimental Medicine, Hungarian Academy of Sciences, Hungary; 2 MTA-SZTE Research Group for Cortical Microcircuits, Department of Physiology, Anatomy and Neuroscience, University of Szeged, Hungary; 3 Institute for Stroke and Dementia Research, Ludwig-Maximilians-University, Munich, Germany; 4 “Momentum” Laboratory of Molecular Neurobiology, Institute of Experimental Medicine, Hungarian Academy of Sciences, Hungary; 5 MTA-DE Cerebrovascular -

Tecnicas Microscopicas
CAP 1: TÉCNICAS MICROSCÓPICAS TÉCNICAS MICROSCÓPICAS 11 Lic. Carlos R. Neira Montoya Lic. Eduardo Sedano Gelvet Lic. María Elena Vilcarromero V. El estudio de los tejidos tal como los observamos hoy en día no sería posible sin la ayuda de la histotecnología; esta disciplina se encarga del estudio de los métodos técnicas y procedimientos que permiten la transformación de un órgano en una película lo suficientemente transparente y contrastada que nos permite su observación a través del microscopio (Fig. 1-1). Para que esto ocurra se tiene que seguir una serie de pasos. Cada uno de estos pasos permite la observación de las características morfológicas del tejido que nos indica la normalidad o la alteración patológica; sin embargo en estudios mucho más minuciosos, estos pasos se harán en función de las estructuras o sustancias que se deseen investigar en la muestra correspondiente. Figura 1-1. Un órgano es transformado en una película transparente. - Pág. 5 - CAP 1: TÉCNICAS MICROSCÓPICAS Los pasos de las técnicas microscópicas para obtener un preparado histológico permanente (láminas) son: 1. Toma de la muestra. 2. Fijación. 3. Inclusión. 4. Microtomía. 5. Coloración. 1. TOMA DE LA MUESTRA Es el momento que se selecciona el órgano o tejido a estudiar. De tres fuentes puede provenir el material humano: las necropsias, las biopsias y las piezas operadas. De éstas, sólo la primera puede darnos material normal; las dos últimas habitualmente proporcionarán tejidos para estudio histopatológico. - Necropsias: son las piezas que se obtienen de un cadáver. Para histología normal es necesario que se trate de un cadáver fresco y que no haya sido atacado por ninguna lesión, por lo menos el órgano que se quiere estudiar. -

NEURAL CONNECTIONS: Some You Use, Some You Lose
NEURAL CONNECTIONS: Some You Use, Some You Lose by JOHN T. BRUER SOURCE: Phi Delta Kappan 81 no4 264-77 D 1999 . The magazine publisher is the copyright holder of this article and it is reproduced with permission. Further reproduction of this article in violation of the copyright is prohibited JOHN T. BRUER is president of the James S. McDonnell Foundation, St. Louis. This article is adapted from his new book, The Myth of the First Three Years (Free Press, 1999), and is reprinted by arrangement with The Free Press, a division of Simon Schuster Inc. ©1999, John T. Bruer . OVER 20 YEARS AGO, neuroscientists discovered that humans and other animals experience a rapid increase in brain connectivity -- an exuberant burst of synapse formation -- early in development. They have studied this process most carefully in the brain's outer layer, or cortex, which is essentially our gray matter. In these studies, neuroscientists have documented that over our life spans the number of synapses per unit area or unit volume of cortical tissue changes, as does the number of synapses per neuron. Neuroscientists refer to the number of synapses per unit of cortical tissue as the brain's synaptic density. Over our lifetimes, our brain's synaptic density changes in an interesting, patterned way. This pattern of synaptic change and what it might mean is the first neurobiological strand of the Myth of the First Three Years. (The second strand of the Myth deals with the notion of critical periods, and the third takes up the matter of enriched, or complex, environments.) Popular discussions of the new brain science trade heavily on what happens to synapses during infancy and childhood. -

Imaging and Quantifying Ganglion Cells and Other Transparent Neurons in the Living Human Retina
Imaging and quantifying ganglion cells and other transparent neurons in the living human retina Zhuolin Liua,1, Kazuhiro Kurokawaa, Furu Zhanga, John J. Leeb, and Donald T. Millera aSchool of Optometry, Indiana University, Bloomington, IN 47405; and bPurdue School of Engineering and Technology, Indiana University–Purdue University Indianapolis, Indianapolis, IN 46202 Edited by David R. Williams, University of Rochester, Rochester, NY, and approved October 18, 2017 (received for review June 30, 2017) Ganglion cells (GCs) are fundamental to retinal neural circuitry, apoptotic GCs tagged with an intravenously administered fluores- processing photoreceptor signals for transmission to the brain via cent marker (14), thus providing direct monitoring of GC loss. The their axons. However, much remains unknown about their role in second incorporated adaptive optics (AO)—which corrects ocular vision and their vulnerability to disease leading to blindness. A aberrations—into SLO sensitive to multiply-scattered light (12). major bottleneck has been our inability to observe GCs and their This clever combination permitted imaging of a monolayer of GC degeneration in the living human eye. Despite two decades of layer (GCL) somas in areas with little or no overlying nerve fiber development of optical technologies to image cells in the living layer (NFL) (see figure 5, human result of Rossi et al.; ref. 12). By human retina, GCs remain elusive due to their high optical trans- contrast, our approach uses singly scattered light and produces lucency. Failure of conventional imaging—using predominately sin- images of unprecedented clarity of translucent retinal tissue. This gly scattered light—to reveal GCs has led to a focus on multiply- permits morphometry of GCL somas across the living human ret- scattered, fluorescence, two-photon, and phase imaging techniques ina.