Removal of Zinc Ions Using Hydroxyapatite and Study of Ultrasound Behavior of Aqueous Media
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
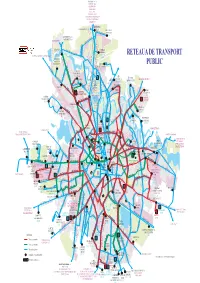
RETEA GENERALA 01.07.2021.Cdr
OTOPENI 780 783 OSTRATU R441 OTOPENI R442 PERIS R443 PISCU R444 GRUIU R446 R447 MICSUNESTII MARI R447B MOARA VLASIEI R448 SITARU 477 GREENFIELD STRAULESTI 204 304 203 204 Aleea PrivighetorilorJOLIE VILLE BANEASA 301 301 301 GREENFIELD 204 BUFTEA R436 PIATA PRESEI 304 131 Str. Jandarmeriei261 304 STRAULESTI Sos. Gh. Ionescu COMPLEX 97 204 205 304 261 Sisesti BANEASA RETEAUA DE TRANSPORT R402 205 131 261 335 BUFTEA GRADISTEA SITARU R402 261 205 R402 R436 Bd. OaspetilorStr. Campinita 361 605 COMPLEX 112 205 261 97 131 261301 COMERCIAL Sos. Bucuresti Ploiesti PUBLIC COLOSSEUM CARTIER 231 Sos. Chitilei Bd. Bucurestii Noi Sos. Straulesti R447 R447B R448 R477 203 335 361 605 780 783 112 R441 R442 R443 R444HENRI R446 COANDA 231 Bd. Aerogarii R402 97 605 231 112 112 CARTIER 112 301 112 DAMAROAIA 131 R436 335 231 Sos. Chitilei R402 24 331R436 CFR Str. Alex. Serbanescu 112 CONSTANTA CARTIER MERII PETCHII R409 112 DRIDU Str. N. Caramfil R402 Bd. Laminorului AUTOBAZA ANDRONACHE 331 65 86 112 135 243 Bd. NORDULUI112 301 382 Bd. Gloriei24 Str. Jiului 605 Sos. 112Pipera 135 Sos. Chitilei Poligrafiei PIATA PLATFORMA Bd. BucurestiiPajurei Noi 231 243 Str. Peris MEZES 780 783 INDUSTRIALA Str. PRESEI Str.Oi 3 45 65 86 331 243 3 45 382 PASAJ Sos. Bucuresti Ploiesti 3 41 243 PIPERA 382 DEPOUL R447 R447BR448 R477 112 231 243 i 65 86 97 243 16 36 COLENTINA 131105 203 205 261203 304 231 261 304 330 135 343 n tuz BUCURESTII NOI a R441 R442 R443 c 21 i CARTIER 605 tr 231R441 361 R442 783 R443 R444 R446 DEPOUL Bd. -

Bucharest Booklet
Contact: Website: www.eadsociety.com Facebook: www.facebook.com/EADSociety Twitter (@EADSociety): www.twitter.com/EADSociety Instagram: https://www.instagram.com/eadsociety/ Google+: www.google.com/+EADSociety LinkedIn: www.linkedin.com/company/euro-atlantic- diplomacy-society YouTube: www.youtube.com/c/Eadsociety Contents History of Romania ………………………………………………………………………………………………………………………………………………….3 What you can visit in Bucharest ……………………………………………………………………………………………………………………………………..4 Where to Eat or Drink ……………………………………………………………………………………………………………………………………………….8 Night life in Bucharest ……………………………………………………………………………………………………………………………………………….9 Travel in Romania ……………………………………………………………………………………………………………………………………………….....10 Other recommendations …………………………………………………………………………………………………………………………………………….11 BUCHAREST, ROMANIA MIDDLE AGES MODERN ERA Unlike plenty other European capitals, Bucharest does not boast of a For several centuries after the reign of Vlad the Impaler, millenniums-long history. The first historical reference to this city under Bucharest, irrespective of its constantly increasing the name of Bucharest dates back to the Middle Ages, in 1459. chiefdom on the political scene of Wallachia, did undergo The story goes, however, that Bucharest was founded several centuries the Ottoman rule (it was a vassal of the Empire), the earlier, by a controversial and rather legendary character named Bucur Russian occupation, as well as short intermittent periods of (from where the name of the city is said to derive). What is certain is the Hapsburg -

LIST of HOSPITALS, CLINICS and PHYSICIANS with PRIVATE PRACTICE in ROMANIA Updated 04/2017
LIST OF HOSPITALS, CLINICS AND PHYSICIANS WITH PRIVATE PRACTICE IN ROMANIA Updated 04/2017 DISCLAIMER: The U.S. Embassy Bucharest, Romania assumes no responsibility or liability for the professional ability or reputation of, or the quality of services provided by the medical professionals, medical facilities or air ambulance services whose names appear on the following lists. Names are listed alphabetically, and the order in which they appear has no other significance. Professional credentials and areas of expertise are provided directly by the medical professional, medical facility or air ambulance service. When calling from overseas, please dial the country code for Romania before the telephone number (+4). Please note that 112 is the emergency telephone number that can be dialed free of charge from any telephone or any mobile phone in order to reach emergency services (Ambulances, Fire & Rescue Service and the Police) in Romania as well as other countries of the European Union. We urge you to set up an ICE (In Case of Emergency) contact or note on your mobile phone or other portable electronics (such as Ipods), to enable first responders to get in touch with the person(s) you designated as your emergency contact(s). BUCHAREST Ambulance Services: 112 Private Ambulances SANADOR Ambulance: 021-9699 SOS Ambulance: 021-9761 BIOMEDICA Ambulance: 031-9101 State Hospitals: EMERGENCY HOSPITAL "FLOREASCA" (SPITALUL DE URGENTA "FLOREASCA") Calea Floreasca nr. 8, sector 1, Bucharest 014461 Tel: 021-599-2300 or 021-599-2308, Emergency line: 021-962 Fax: 021-599-2257 E-mail: [email protected] Website: http://www.urgentafloreasca.ro Medical Director: Dr. -

Soil Mite Communities (Acari: Mesostigmata) As Indicators of Urban Ecosystems in Bucharest, Romania M
www.nature.com/scientificreports OPEN Soil mite communities (Acari: Mesostigmata) as indicators of urban ecosystems in Bucharest, Romania M. Manu1,5*, R. I. Băncilă2,3,5, C. C. Bîrsan1, O. Mountford4 & M. Onete1 The aim of the present study was to establish the efect of management type and of environmental variables on the structure, abundance and species richness of soil mites (Acari: Mesostigmata) in twelve urban green areas in Bucharest-Romania. Three categories of ecosystem based upon management type were investigated: protected area, managed (metropolitan, municipal and district parks) and unmanaged urban areas. The environmental variables which were analysed were: soil and air temperature, soil moisture and atmospheric humidity, soil pH and soil penetration resistance. In June 2017, 480 soil samples were taken, using MacFadyen soil core. The same number of measures was made for quantifcation of environmental variables. Considering these, we observed that soil temperature, air temperature, air humidity and soil penetration resistance difered signifcantly between all three types of managed urban green area. All investigated environmental variables, especially soil pH, were signifcantly related to community assemblage. Analysing the entire Mesostigmata community, 68 species were identifed, with 790 individuals and 49 immatures. In order to highlight the response of the soil mite communities to the urban conditions, Shannon, dominance, equitability and soil maturity indices were quantifed. With one exception (numerical abundance), these indices recorded higher values in unmanaged green areas compared to managed ecosystems. The same trend was observed between diferent types of managed green areas, with metropolitan parks having a richer acarological fauna than the municipal or district parks. -

Locatii Call Center Adresa Aviatiei 021 9268 Floreasca Park , Soseaua
RETEAUA REGINA MARIA Locatii Call Center Adresa Policlinici Bucuresti Aviatiei 021 9268 Floreasca Park , Soseaua Pipera, nr. 43, Sector 1 Baneasa 021 9268 Str. Ion Ionescu de la Brad, nr. 5B, Sector 1 BBP 021 9268 Șos. București - Ploiești, nr. 1A, corp A, parter, Sector 1 Cotroceni 021 9268 Șos. Cotroceni, nr. 20, Sector 6 Dorobanti 021 9268 Calea Dorobanți, nr. 240, Sector 1 Enescu 021 9268 Str. George Enescu, nr. 12, Sector 1 Floreasca 021 9268 Str. Hatmanul Arbore, nr. 15-19, Sector 1 Lujerului 021 9268 Bd. Iuliu Maniu, nr. 51, Sector 6 Opera 021 9268 Str. Costache Negri, nr. 1-5, Sector 5 Pipera 021 9268 Str. Dimitrie Pompeiu, nr. 9-9A, clădirea 16, parter, Sector 2 Sun Plaza 021 9268 Calea Văcărești, nr. 391, S4, birouri mall SunPlaza, etaj 2, Sector 4 Titu Maiorescu 021 9268 Calea Văcărești, nr. 189, (în interiorul Universității Titu Maiorescu, corp M), Sector 4 - Victoria 021 9268 Str. Buzesti nr. 75-77, etaj 3, sector 1, Bucuresti Doamna Ghica 021 9268 Str. Doamna Ghica nr 135, sector 2, Bucuresti Perla 021 9268 Calea Dorobantilor Nr. 126 - 130 Sema Park 021 9268 Splaiul Independentei, nr. 319, parter, sector 6, Bucuresti Policlinici tara Bacau 021 9268 Calea Romanului, nr. 39, Bacău Brasov 021 9268 Bd. Iuliu Maniu, nr. 49 Brasov Calea Bucuresti 021 9268 Calea Bucuresti Nr. 30 Brasov Centrul Civic 021 9268 Str. Mihail Kogalniceanu Nr. 16 Cluj Muzeului 021 9268 Strada Sextil Puscariu, Nr. 2 Cluj Pasteur 021 9268 Str. Louis Pasteur, nr. 24/72, et.1 Cluj Observatorului 021 9268 Str. -

Noapte (Hartă)
ROND ODAII N117 N117 Bd. Gh. Ionescu Sisesti COMPLEX RETEAUA DE NOAPTE N118 COMERCIAL N117 N118 n BANEASA n Io es r. Io cu N118 St N113 d Bd. Bucurestii Noi e l N118 a Sos. Bucuresti Ploiesti B N118 r ad N113 N117 AUTOBAZA AUTOBAZA NORDULUI PIPERA Str. CoralilorN113 Str. Av. Serbanescu C.F.R. N118 CONSTANTA CARTIER Bd. Laminorului N119 BANEASA Bd. GlorieiN113 Sos. Chitilei N113 Sos. PiperaN119 PASAJ N118 N113 N117 N118 COLENTINA DEPOUL N101 N108 N123 BUCURESTII NOI a c DEPOUL DEPOUL s a e COLENTINA GIULESTI r o l F N119 . CARTIER l ColentinaN123 Cal. Giulesti a Sos. Fundeni 16 FEBRUARIE C Sos. N108 N110 N110 Bd. Ion N113 Cal. Grivitei N123 N101 N101 N117 N118 AUTOBAZA Mihalache N113 FLOREASCA Str. B Cal. Giulesti Bd. Lacul Tei N113 d N123 Doamna . N113 C N113 N108 o N123 n N118 N110 sN118 DEPOUL Ghica t ru VICTORIA c N119 to N101 N108 ri N113 lo Sos.Crangasi Sos. Colentina r N113 Sos. Stefan cel N118 N117 Mare N119 N113 BAICULUI N108 Cal. Grivitei N125 N119 N125 N125 N101 N125 Sos.Virtutii N N108 N110 N118 N123 108 Bd. N. Balcescu Str.N123 Viitorului N108Sos. Pantelimon N117 N117 Bd. Dacia Splaiul N117 N119 Bd. Ferdinand N117 N101 N101 Sos. Pantelimon Independentei N117 Calea MosilorN123 N101 N102 108 N113 N123 N113 N125 GRANITUL GRUP SCOLAR N N102 AUTO Bd. Pache ProtopopescuN113 N113 Sos. Vergului N115 N110 N102 Str. Vatra Luminoasa Sos. MihaiN102 N115 N116 AUTOBAZA DEPOUL Bd. Bd. Basarabia Sos. Garii Catelu N110 N118 Sos.Virtutii N116 Bd. -

(In)Justice and Protest in Bucharest After the Colectiv Fire
This is a repository copy of Corruption and Conflagration: (In)Justice and Protest in Bucharest after the Colectiv Fire. White Rose Research Online URL for this paper: https://eprints.whiterose.ac.uk/151702/ Version: Accepted Version Article: Creţan, Remus and O'Brien, Thomas Anthony orcid.org/0000-0002-5031-736X (2020) Corruption and Conflagration: (In)Justice and Protest in Bucharest after the Colectiv Fire. URBAN GEOGRAPHY. pp. 368-388. ISSN 0272-3638 https://doi.org/10.1080/02723638.2019.1664252 Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ This is an Accepted Manuscript of an article published by Taylor & Francis in Urban Geography on 3 October 2019, available online: https://doi.org/10.1080/02723638.2019.1664252 Corruption and conflagration: (In)Justice and protest in Bucharest after the Colectiv fire The fire in the Colectiv nightclub in Bucharest, Romania in October 2015 led to sustained, nationwide protests that forced the resignation of the government. -

Physical Properties of the Quaternary Sedimentary Rocks in the Eastern Bucharest Area
Romanian Reports in Physics, Vol. 57, No. 1, P. 151–163, 2005 PHYSICAL PROPERTIES OF THE QUATERNARY SEDIMENTARY ROCKS IN THE EASTERN BUCHAREST AREA ANDREI BALA1, VICTOR RAILEANU1, NICOLAE MANDRESCU1, ION ZIHAN2, EUGEN DANANAU2 1 National Institute of Research and Development for Earth Physics, P.O. Box MG-2, Bucharest-Magurele, Romania, [email protected] 2 “Prospecþiuni” S.A., str. Caransebeº, nr. 1, Bucharest, Romania (Received October 4, 2004) Abstract. New seismic measurements are performed in the eastern Bucharest area with the purpose of defining better the physical properties of the shallow sedimentary rocks. A high resolution seismic profile, 600 m in length, was carried out in the area, as well as down-hole seismic measurements in two boreholes drilled at the ends of the profile. Computing and interpretation of the data lead to the conclusion that shallow sedimentary rocks can be considered weak and very weak in the area, down to 70 m depth. Seismic wave velocity values and bulk density values presented in the paper associated with local geology are useful primary data in the seismic microzonation of Bucharest City. This work was performed in the frame of the CERES Contract no. 34/11.11.2002, funded by the Ministry of Education and Research, Bucharest, Romania. Key words: seismic profile, down-hole seismic measurements, seismic microzonation. 1. GEOMORPHOLOGY OF THE BUCHAREST AREA Bucharest is situated in the central part of the Romanian Plain, a transitional area between the piedmont plains from the north and the Danube River to the south [1]. Some local plains can be distinguished in the city area: Bãneasa – Pantelimon, Giuleºti – Floreasca, Vergului and Cotroceni – Vãcãreºti (Fig. -

Floreasca Park Completed 2013 31 Awards
Content 0 2 location 0 4 concept 1 4 site plan 1 6 building b 1 8 building c 2 4 landscaping 2 6 specifications 2 8 the development team 2 9 completed office developments by Portland Trust 3 2 awards For a richer experience scan the QR code: AVAILABLE ON THE AVAILABLE ON THE All movements Location on on scheme ra Pipe aua Sose Capit an Al e xandru S ty erbane Ci From scu entre C ompeiu Pipera City e P To Green Bucharest court Dimitri One Barbu V Aurel a Vlaiicuc care s c u Aproximate travel times to/from Oregon Park: City Centre by car: 15 mins City Centre by bus: 20 mins City Centre by metro: 15 mins Airport by car: 25 mins Oregon Park is located just North of Bucharest city centre. The area around Floreasca/Barbu Vacarescu has become one of the most popular suburban office zones in the city. It has a pleasant environment near the lakes, parks and sports facilities and has excellent transport connections. There are now many multinational companies located around Calea Floreasca, Barbu Vacarescu and Dimitrie Pompeiu, especially in the business support, IT and high tech sectors which continue to grow. There are retail facilities and restaurants available at the Promenada Shopping Centre which opened in 2014. 02 Under Lac Străuleşti construction Aeroportul Laminorului Henry Coandă Under Parc Bazilescu Aeroportul Aurel construction Băneasa Vlaicu Pipera Jiului Under 1 Mai design Piata Gara Aviatorilor Griviţa Presei de nord Libere Piaţa Victoriei Basarab Ştefan cel Mare Crângaşi Obor Petrache Piaţa Romană Poenaru Pantelimon Grozăveşti Păcii -

Romania Market Overview Refreshed Real-Estate Perspectives 2016 –2017 Romania Market Overview 2016–2017
ROMANIA MARKET OVERVIEW REFRESHED REAL-ESTATE PERSPECTIVES 2016 –2017 ROMANIA MARKET OVERVIEW 2016–2017 2016 was a record year for the Bucharest It takes approximately 10 years office market, with take�up reaching for a typical Bucharester to buy almost 350,000 sq m, 41% higher than a new two room apartment. in 2015. The year brought a significant There were approximately 10,000 volume of new deliveries – approximately residential transactions for new CONTENTS 300,000 sq m. Demand in 2016 was apartments in 2016 in Bucharest 50% higher than in the boom years. and surrounding areas. 04 ROMANIAN ECONOMIC OVERVIEW Office space goes back to nature, where human 06 OFFICE MARKET beings feel most at The total investment OFFICE RESIDENTIAL MARKET MARKET home and can reach volume reached their most efficient and €705 million in 2016, effective states. 10 INVESTMENT MARKET almost 16% higher than 2015. Foreign funds accounted for more than 99% of the total activity. 12 RETAIL MARKET 14 LAND MARKET INVESTMENT PROJECT MANAGEMENT MARKET 16 INDUSTRIAL & LOGISTICS MARKET HIGHLIGHTS 18 RESIDENTIAL MARKET 20 PROJECT MANAGEMENT 21 PROPERTY TAXATION INDUSTRIAL RETAIL MARKET MARKET 22 LEGAL ASPECTS Industrial take�up across the country LAND Fashion retailers maintained in 2016 reached record levels with MARKET a competitive pace in 2016, 415,000 sq m of space absorbed. supported by the opening of In Bucharest, total take�up in 2016 new malls and the expansion was close to 280,000 sq m, which was of existing ones. approximately 85% increase compared with 2015, and a record year. The key players on the industrial market are looking to secure new land plots for development and their interest has moved to the northern areas: the DN1–A3 and A2–A3 city ring segments have land plots with all utilities in place and ready to start construction work. -

Strategia Locală Integrată De Dezvoltare Durabilă a Sectorului 6 București
Strategia locală integrată de dezvoltare durabilă a Sectorului 6 București Cuprins I. Introducere .......................................................................................................................................................5 II. ANALIZA ECONOMICO - SOCIALĂ A SECTORULUI 6............................................................................. 10 II.1. Coordonarea cu alte strategii și planuri relevante ................................................................................ 10 II. 2. Contextul regional și urban .................................................................................................................... 16 II.2.1 Analiza SWOT a contextului regional și urban........................................................................... 18 II. 3. Definirea teritoriului ................................................................................................................................ 20 II.3.1. Așezarea geografică ...................................................................................................................... 20 II.3.2. Scurt istoric ..................................................................................................................................... 21 II.3.3 Relieful............................................................................................................................................... 40 II.3.4. Condiţii climatice ........................................................................................................................... -

Romania MARKET Overview Annual Review & Outlook
2011 Romania MARKET Overview Annual review & outlook Highlights • Activity in the Bucharest office market improved in 2010, as take-up was recorded at 225,000 sq m, an increase of 125% on 2009. Prime office rents were broadly stable during the second half of the year, after falling slightly in the first six months. • The high street segment was the most active in the retail market during 2010. Strong demand for retail space in Bucharest has helped prime high street rents to stabilize, though rents in secondary locations remain under downward pressure. • Vacancy rates in the industrial sector have risen, despite improved demand and limited development completions. Prime industrial rents fell by 10-20% during 2010. • The Romanian investment market experienced a modest recovery in 2010, with approximately €500 million being invested. However, activity remains constrained by difficult access to finance. 2 www.knightfrank.com Contents 1. Office Market 4 2. Retail Market 9 3. Industrial Market 12 4. Land Market 14 5. Investment Market 15 6. Investment Property Accounting and Tax Matters 17 3 2011 Romania MARKET OVERVIEW Annual review & outlook http://knightfrank.com/romania North Point, with 28,000 sq m GLA, developed by Sity International. Exclusive project of The Advisers/Knight Frank In comparison with the previous year, 2010 was characterized by a resurgence of the office market in Bucharest. While there were fewer deliveries of new office space than in 2009, take-up 1. OFFICE improved and 2010 brought an increase of 125% in the amount MARKET of leased space. Supply Over 70% of the space delivered was in the central and semi-central areas, while the In 2010, the Bucharest market saw the periphery registered only 29% of the total.