Ornithorhynchus Anatinus): a BIBLIOGRAPHY
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Oldest Platypus and Its Bearing on Divergence Timing of the Platypus and Echidna Clades
The oldest platypus and its bearing on divergence timing of the platypus and echidna clades Timothy Rowe*†, Thomas H. Rich‡§, Patricia Vickers-Rich§, Mark Springer¶, and Michael O. Woodburneʈ *Jackson School of Geosciences, University of Texas, C1100, Austin, TX 78712; ‡Museum Victoria, PO Box 666, Melbourne, Victoria 3001, Australia; §School of Geosciences, PO Box 28E, Monash University, Victoria 3800, Australia; ¶Department of Biology, University of California, Riverside, CA 92521; and ʈDepartment of Geology, Museum of Northern Arizona, Flagstaff, AZ 86001 Edited by David B. Wake, University of California, Berkeley, CA, and approved October 31, 2007 (received for review July 7, 2007) Monotremes have left a poor fossil record, and paleontology has broadly affect our understanding of early mammalian history, been virtually mute during two decades of discussion about with special implications for molecular clock estimates of basal molecular clock estimates of the timing of divergence between the divergence times. platypus and echidna clades. We describe evidence from high- Monotremata today comprises five species that form two resolution x-ray computed tomography indicating that Teinolo- distinct clades (16). The echidna clade includes one short-beaked phos, an Early Cretaceous fossil from Australia’s Flat Rocks locality species (Tachyglossus aculeatus; Australia and surrounding is- (121–112.5 Ma), lies within the crown clade Monotremata, as a lands) and three long-beaked species (Zaglossus bruijni, Z. basal platypus. Strict molecular clock estimates of the divergence bartoni, and Z. attenboroughi, all from New Guinea). The between platypus and echidnas range from 17 to 80 Ma, but platypus clade includes only Ornithorhynchus anatinus (Austra- Teinolophos suggests that the two monotreme clades were al- lia, Tasmania). -
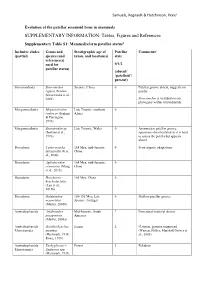
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus -

Constituents of Platypus and Echidna Milk, with Particular Reference to the Fatty Acid Complement of the Triglycerides
Aust. J. Bioi. Sci., 1984, 37, 323-9 Constituents of Platypus and Echidna Milk, with Particular Reference to the Fatty Acid Complement of the Triglycerides Mervyn Grijfiths,A,B Brian Green,A R. M. C. Leckie,A Michael Messerc and K. W. NewgrainA A Division of Wildlife and Rangelands Research, CSIRO, P.O. Box 84, Lyneham, A.C.T. 2602. B Department of Physical Biochemistry, Australian National University, G.P.O. Box 334, Canberra, A.C.T. 2601. cDepartment of Biochemistry, University of Sydney, Sydney, N.S.W. 2006. Abstract The mature milk of platypuses (Ornithorhynchus anatinus) exhibit 39· 1 gf 100 g solids, of which crude lipid accounts for 22·2% crude protein 8·2%, hexose 3·3% and sialic acid 0·43%; iron is in high concentration, 21·1 mgfl. Echidna (Tachyglossus aculeatus) milk is more concentrated, containing 48·9% solids, with lipid. protein, hexose and sialic acid accounting for 31 ·0, 12·4, 1· 6 and O· 70% respectively; it is even richer in iron, 33·3 mgfl. The fatty acid complement of milk triglyceride from platypuses living in their natural habitat is different from that of the echidna living in the wild: platypus milk triglyceride contains 17·6% palmitic, 25 . 2% oleic and c. 30% long-chain polyunsaturated acids, whereas that of echidnas exhibits 15· 9% palmitic, 61 ·2% oleic and only c. 7% polyunsaturated acids. The fatty acid complement of echidna milk triglyceride can be changed by altering the fatty acid complement of the dietary lipid. Introduction Levels of the proximate constituents, i.e. total solids, crude lipid, crude protein, carbohydrates and minerals, were determined in samples of platypus and echidna milk. -

Brief Report Vol
Brief report Vol. 46, No. 1, pp. 113-1 18, Warszawa 2001 Monotreme nature of the Australian Early Cretaceous mammal Teinolophos THOMAS H. RICH, PATRICIA VICKERS-RICH, PETER TRUSLER, TIMOTHY F. FLANNERY, RICHARD CIFELLI, ANDREW CONSTANTINE, LESLEY KOOL, and NICHOLAS VAN KLAVEREN The morphology of the single preserved molar of the holotype of the Australian Early Creta- ceous (Aptian) mammal Teinolophos trusleri shows that it is a monotreme and probably a steropodontid, rather than a 'eupantothere' as originally proposed. The structure of the rear of the jaw of T. trusleri supports the molecular evidence that previously formed the sole basis for recognising the Steropodontidae as a distinct family. When the holotype of Teinolophos trusleri was first described from the Early Cretaceous (Aptian) Strzelecki Group of southern Victoria, Australia (Rich et al. 1999), it was regarded as a member of the Order Eupantotheria Kermack & Mussett, 1958 (= Legion Cladotheria McKenna, 1975 - Infralegion Tribosphenida McKenna, 1975) of uncertain family. This interpretation was based in large part on the inferred structure of the penultimate lower molar, the only tooth preserved on the se- verely crushed holotype. The crown of that tooth was largely obscured by a hard matrix. As a conse- quence of that, a critical misidentification of the cusp in the posterolingual region of the tooth as the metaconid rather than the hypoconulid was made. It was this erroneous interpretation and the conse- quent corollaries that the trigonid was anteroposteriorlyexpanded and the talonid unbasined that led Rich et al. (1999) to intepret the specimen as a 'eupantothere'. In September 1999, Mr. Charles Schaff of Harvard University successfully cleared the obscuring matrix from crown of the tooth (Fig. -

Mammal Disparity Decreases During the Cretaceous Angiosperm Radiation
Mammal disparity decreases during the Cretaceous angiosperm radiation David M. Grossnickle1 and P. David Polly2 1Department of Geological Sciences, and 2Departments of Geological Sciences, Biology, and Anthropology, rspb.royalsocietypublishing.org Indiana University, Bloomington, IN 47405, USA Fossil discoveries over the past 30 years have radically transformed tra- ditional views of Mesozoic mammal evolution. In addition, recent research provides a more detailed account of the Cretaceous diversification of flower- Research ing plants. Here, we examine patterns of morphological disparity and functional morphology associated with diet in early mammals. Two ana- Cite this article: Grossnickle DM, Polly PD. lyses were performed: (i) an examination of diversity based on functional 2013 Mammal disparity decreases during dental type rather than higher-level taxonomy, and (ii) a morphometric analysis of jaws, which made use of modern analogues, to assess changes the Cretaceous angiosperm radiation. Proc R in mammalian morphological and dietary disparity. Results demonstrate a Soc B 280: 20132110. decline in diversity of molar types during the mid-Cretaceous as abundances http://dx.doi.org/10.1098/rspb.2013.2110 of triconodonts, symmetrodonts, docodonts and eupantotherians dimin- ished. Multituberculates experience a turnover in functional molar types during the mid-Cretaceous and a shift towards plant-dominated diets during the late Late Cretaceous. Although therians undergo a taxonomic Received: 13 August 2013 expansion coinciding with the angiosperm radiation, they display small Accepted: 12 September 2013 body sizes and a low level of morphological disparity, suggesting an evol- utionary shift favouring small insectivores. It is concluded that during the mid-Cretaceous, the period of rapid angiosperm radiation, mammals experi- enced both a decrease in morphological disparity and a functional shift in dietary morphology that were probably related to changing ecosystems. -

Three Types of Mammals by Cindy Grigg
Three Types of Mammals By Cindy Grigg 1 Earth has a large variety of animals living on it. Scientists classify animals into groups based on common characteristics. Mammals are a group of animals (vertebrates) that have backbones and hair or fur. They are warm-blooded (endothermic), and they have four-chambered hearts. They also feed their young with milk from the mother's body. The young of most mammals are born alive. 2 Mammals have about six thousand different species, or kinds, of animals in their group or class. Mammals can be divided into three more groups based on how their babies develop. These three groups are monotremes, marsupials, and the largest group, placental mammals. 3 Monotremes are mammals that lay eggs. The only monotremes that are alive today are the spiny anteater, or echidna, and the platypus. They live in Australia, Tasmania, and New Guinea. These mammals are really different from other mammals. Their body temperature is lower than most warm-blooded animals, a feature that has more in common with reptiles. Their name comes from the fact that they have only one body opening for both wastes and eggs to pass through. Echidnas have sharp spines scattered throughout their hair. They look like a spiky ball. The female anteater lays usually one leathery-shelled egg directly into the pouch on her belly. The egg hatches after only ten or eleven days. The newborn baby is tiny, about the size of a dime. After the baby hatches, it stays in the pouch for several weeks and continues to develop. -

17. Morphology and Physiology of the Metatheria
FAUNA of AUSTRALIA 17. MORPHOLOGY AND PHYSIOLOGY OF THE METATHERIA T.J. DAWSON, E. FINCH, L. FREEDMAN, I.D. HUME, MARILYN B. RENFREE & P.D. TEMPLE-SMITH 1 17. MORPHOLOGY AND PHYSIOLOGY OF THE METATHERIA 2 17. MORPHOLOGY AND PHYSIOLOGY OF THE METATHERIA EXTERNAL CHARACTERISTICS The Metatheria, comprising a single order, Marsupialia, is a large and diverse group of animals and exhibits a considerable range of variation in external features. The variation found is intimately related to the animals' habits and, in most instances, parallels that are found in the Eutheria. Useful general references to external characteristics include Pocock (1921), Jones (1923a, 1924), Grassé (1955), Frith & Calaby (1969), Ride (1970) and Strahan (1983). Body form In size, the marsupials range upwards from the Long-tailed Planigale, Planigale ingrami, a small, mouse-like animal weighing only around 4.2 g, with a head- body length of 59 mm and a tail 55 mm long. At the other extreme, there are large kangaroos, such as the Red Kangaroo, Macropus rufus, in which the males may weigh as much as 85 kg and attain a head-body length of 1400 mm and a tail of 1000 mm. Body shape also varies greatly. The primarily carnivorous marsupials, the dasyurids (for example, antechinuses, dunnarts, quolls, planigales and others), are small to medium sized quadrupeds with subequal limbs. The tail is relatively slender and generally about half the length of the body. The omnivorous peramelids show increased development of the hind limbs in keeping with their rapid bounding locomotion. Saltatory or hopping forms (for example kangaroos and wallabies), carry the hind limb specialisation to an extreme, with a concomitant reduction of the forelimbs (Fig. -

The Miocene Mammal Necrolestes Demonstrates the Survival of a Mesozoic Nontherian Lineage Into the Late Cenozoic of South America
The Miocene mammal Necrolestes demonstrates the survival of a Mesozoic nontherian lineage into the late Cenozoic of South America Guillermo W. Rougiera,b,1, John R. Wibleb, Robin M. D. Beckc, and Sebastian Apesteguíad,e aDepartment of Anatomical Sciences and Neurobiology, University of Louisville, Louisville, KY 40202; bSection of Mammals, Carnegie Museum of Natural History, Pittsburgh, PA 15206; cSchool of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW, Australia; dCEBBAD–Fundación de Historia Natural ‘Félix de Azara’, Universidad Maimónides, 1405 Buenos Aires, Argentina; and eConsejo Nacional de Investigaciones Científicas y Técnicas de Argentina, C1033AAJ Buenos Aires, Argentina Edited by Richard L. Cifelli, University of Oklahoma, Norman, OK, and accepted by the Editorial Board October 18, 2012 (received for review July 27, 2012) The early Miocene mammal Necrolestes patagonensis from Pata- not referable to either Metatheria or Eutheria, but did not discuss gonia, Argentina, was described in 1891 as the only known extinct the evidence for this interpretation, nor did they identify the placental “insectivore” from South America (SA). Since then, and specific therian lineages they considered to be potential relatives despite the discovery of additional well-preserved material, the of Necrolestes. Starting in 2007, we oversaw additional prepara- systematic status of Necrolestes has remained in flux, with earlier tion of Necrolestes specimens that comprise the best-preserved studies leaning toward placental affinities and more recent ones material currently available, including skulls, jaws, and some iso- endorsing either therian or specifically metatherian relationships. We lated postcranial bones; as a result, many phylogenetically signif- have further prepared the best-preserved specimens of Necrolestes icant features have been revealed for the first time. -

The Miraculous Platypus
CSIRO PUBLISHING Foreword www.publish.csiro.au/journals/rfd Reproduction, Fertility and Development, 2009, 21, v–vi The miraculous platypus Roger V. Short Faculty of Medicine, Dentistry and Health Sciences, University of Melbourne, Vic. 3010, Australia. Email: [email protected] The platypus, Ornithorhynchus anatinus, is surely one of the the water, but showed so little of their bodies that they might most amazing animals in the world, and it still has so much to easily have been mistaken for water rats. Mr. Browne shot one; tell us about the whole of mammalian evolution. It is therefore certainly it is a most extraordinary animal; a stuffed specimen particularly appropriate on the 200th anniversary of the birth does not at all give a good idea of the head and beak when fresh; of Charles Darwin in 1809 to recall his first encounter with a the latter becoming hard and contracted’ (Darwin 1839). platypus during the voyage of the Beagle, and show how he was This brief encounter must have made a deep impression on able to use it to support his concept of the gradual evolution of Darwin, because in On the Origin of Species (Darwin 1859) species over time. he describes how: ‘in fresh water we find some of the most The first platypus to come to the attention of the Western anomalous forms known to the world, as the Ornithorhynchus world was the skin, and an accompanying drawing of the animal and Lepidosiren which, like fossils, connect to a certain extent in life, sent by John Hunter, Governor of the Penal Settlement at two Orders at present widely sundered in the natural scale. -

Oxford Word of the Month - November: Platypup Noun: a Baby Platypus
Click here if you are having trouble viewing this message. Share our Word of the Month: Oxford Word of the Month - November: platypup noun: a baby platypus. THE STORY BEHIND THE WORD OF THE MONTH There is some discussion on the Internet about the correct name for a baby platypus. Some commentators note that a baby platypus may be called a puggle, while others say that puggle refers only to a baby echidna. The following writer has an alternative: A common misconception is that a baby platypus is called a puggle. There is no actual official name for a baby platypus, but a common suggested name is ‘platypup’. (Sunshine Coast Sunday, 13 January 2013) The word platypup has received some interest in recent years, including the establishment of a Facebook page to campaign for its official acceptance: ‘Platypup: Give the baby platypus a name’. Platypup has a long but interrupted history. The earliest evidence appears in the 1940s and refers to the first platypus bred in captivity, in a Victorian wildlife sanctuary: A platypup’s birth made history … For more than two months Fleahy restrained his longing to take a peep at the platypup. Then, last Monday, he dug down to the blind end of the burrow, found the nest and brought the youngster up for a quick inspection. (Sydney Sun, 9 January 1944) A year later the same baby platypus is mentioned in several newspaper items: Platy-Pup Is One Year Old … Corrie, the first platypus to be bred in captivity, is one year old. (Brisbane Courier-Mail, 25 January 1945) The ‘Sun’ called the first baby platypus to be bred in captivity a platy-pup . -

Description of a Cranial Endocast from a Fossil Platypus, Obdurodon Dicksoni
JOURNAL OF MORPHOLOGY 267:1000–1015 (2006) Description of a Cranial Endocast From a Fossil Platypus, Obdurodon dicksoni (Monotremata, Ornithorhynchidae), and the Relevance of Endocranial Characters to Monotreme Monophyly Thomas E. Macrini,1* Timothy Rowe,1 and Michael Archer2 1Jackson School of Geosciences and University of Texas High-Resolution X-ray Computed Tomography Facility, University of Texas at Austin, Austin, Texas 78712, USA 2School of Biological Science, University of New South Wales, Sydney, New South Wales 2052, Australia ABSTRACT A digital cranial endocast of the Miocene and have rounded and dorsoventrally compressed platypus Obdurodon dicksoni was extracted from high- bodies that are mostly covered by hollow spines that resolution X-ray computed tomography scans. This endo- are essentially modified hairs. The most prominent cast represents the oldest from an unequivocal member of feature on the echidna head is the elongated, hair- either extant monotreme lineage and is therefore impor- less snout. tant for inferring character support for Monotremata, a clade that is not well diagnosed. We describe the Obdur- Whereas monotreme monophyly is virtually un- odon endocast with reference to endocasts extracted from questioned, there are relatively few unequivocal skulls of the three species of extant monotremes, particu- morphological synapomorphies for Monotremata de- larly Ornithorhynchus anatinus, the duckbill platypus. scribed in the literature; most of these are osteolog- We consulted published descriptions and illustrations of ical (as summarized by Gregory, 1947; Rowe, 1986). whole and sectioned brains of monotremes to determine However, some of these were subsequently shown to which external features of the nervous system are repre- be equivocal. -

16. Ornithorhynchidae
FAUNA of AUSTRALIA 16. ORNITHORHYNCHIDAE T. R. GRANT 1 16. ORNITHORHYNCHIDAE 2 16. ORNITHORHYNCHIDAE DEFINITION AND GENERAL DESCRIPTION Ornithorhynchus anatinus, the Platypus, is the only extant representative of the Ornithorhynchidae, a family which has occupied the Australian mainland for at least 15 million years (Woodburne & Tedford 1975; Archer, Plane & Pledge 1978). It is a small amphibious mammal, which possesses a characteristic pliable duck-like bill and has strongly webbed forefeet. Like its living relatives, the echidnas (Tachyglossus aculeatus and Zaglossus bruijnii), the Platypus is oviparous. HISTORY OF DISCOVERY The first specimen of Ornithorhynchus anatinus (a dried skin) reached Britain in 1798. In spite of some initial consternation over its authenticity, the animal was described by George Shaw in 1799 and named Platypus anatinus. It was redescribed independently as O. paradoxus in 1800, but later, following the rules of priority of nomenclature, became O. anatinus (Shaw) (fide Iredale & Troughton 1934). It was not until 1884 that it was finally concluded that O. anatinus is oviparous (Caldwell 1884b). MORPHOLOGY AND PHYSIOLOGY External Characteristics The Platypus is quite a small animal, although there is considerable size variation between populations in various parts of eastern Australia (Carrick 1983; Grant & Temple-Smith 1983). There is also a notable size difference between the sexes. Males average 500 mm (range 450–600 mm) in total length and weigh 1700 g (1000–2400 g), while females are 430 mm (390–550 mm) and 900 g (700–1600 g), respectively. Fine, dense fur covers the body except for the bill, the complete manus, much of the pes and the underside of the tail.