Platypus Husbandry and Biology Becomes Available
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

2018-21 Strategic Plan
2018-2021 Strategic plan Zoos Victoria Fighting Extinction to secure a future rich in wildlife Conservation Reach Impact Minister for Energy, Kate Vinot, Chair, Zoos Victoria Dr Jenny Gray, CEO, Zoos Victoria Environment and Climate Change, the Hon. Lily D’Ambrosio “Our zoos foster care and “Now, more than ever before, our conservation by connecting commitment is unwaveringly strong “Native wildlife are unique and people with wildlife and our to ensure that “no Victorian terrestrial, precious – and it’s important that commercial activities allow us to vertebrate species will go extinct on Victorians get involved in conserving make meaningful investments our watch.” It’s a rare privilege to and caring for our wildlife. Our zoos to protect our most vulnerable work with such amazing people in our play a vital role in achieving this goal.” species and fight extinction.” joined mission to fight extinction.” Vision Mission As a world leading zoo-based As a world leading zoo-based conservation organisation, we conservation organisation we will will fight extinction to secure a fight wildlife extinction through: future rich in wildlife. • Innovative, scientifically • Strong commercial sound breeding and recovery approaches that secure our programs to support critically financial sustainability; and endangered species; • Profound zoo-based experiences • Amplifying our voice as a that connect people with wildlife trusted champion for wildlife and enrich our world. conservation; ANIMALS CONSERVATION 1 Ensure that our efforts to care for 1 Complete the implementation of and conserve wildlife are justified, Wildlife Conservation Masterplan 1.0. humane and effective. 2 Develop and implement Wildlife 2 Advance staff skills and capacity Conservation Masterplan 2.0. -

2015-16 ANNUAL REPORT Our Vision Is to Be the World’S Leading Zoo-Based Conservation Organisation
A YEAR WITH ZOOS VICTORIA 2015-16 ANNUAL REPORT Our vision is to be the world’s leading zoo-based conservation organisation. We do this by fighting wildlife extinction. Southern Corroboree Frog • Pseudophryne corroboree 2 ZOOS VICTORIA ANNUAL REPORT 2015–16 CONTENTS Chair’s Message 4 CEO’s Message 5 Our Charter and Purpose 6 Fighting Extinction 8 Animals of the Zoo 9 Highlights 2015-16 10 Five Action Areas Conservation 14 Our Animals 20 Visitors and Community 26 Our People 28 Financial Sustainability 30 Organisational Chart 32 Our Workplace Profile 33 Key Performance Indicators 34 Financial Summary 36 Board Attendance 37 Board Profiles 38 Board Committees 40 Corporate Governance and Other Disclosure 41 Our Partners and Supporters 45 Financial Report 49 ZOOS VICTORIA ANNUAL REPORT 2015–16 3 CHAIR’S MESSAGE “ We strive to profoundly influence people to take action to save wildlife.” Anne Ward, Chair Zoos Victoria More people than ever before are The Minute to Midnight Gala Ball was visiting our zoos, with record visitation one such occasion where we engaged at Melbourne Zoo, Healesville Sanctuary an audience not traditionally associated and Werribee Open Range Zoo in 2015-16. with the Zoo. The night showcased Zoos And while we continue to attract Victoria, both as a great place to visit more people through our gates, we and one that is committed to saving continue to change and develop to meet wildlife. the expectations of our visitors. 2015-16 On behalf of the Board, staff and was a year of exploration and reflection animals of Zoos Victoria, I would like at our zoos as we embarked on new to acknowledge the many people and ways to foster deeper connections organisations that have helped make between our visitors and our animals. -

Master Index
To download a printer friendly version of this index, go to www.entertainmentbook.com.au Master Index 365 Roadside Assistance G84 Avis Australia H49-52 529 The Terrace A31 Avoca Beach Picture Theatre E46 Awaba House Café B61 A Awezone Trampoline Park E66 AAT Kings H19, 20 Absolute Thai C9 B ACE H61, 62 Babbingtons Bar and Grill A29 Activate Foods G29 Bakers Delight D7, 8, 9 Adairs F11, 12 The Balcony Restaurant & Bar A28 Adina & Medina Apartment Hotels J29, 30 Balloon Boutique G63 Adnama Beauty Salon G50 Balloon Worx G64 Air New Zealand H7, 8 Bar Depot A20 Ala Moana by Mantra J77, 78 Bar Petite A67 Albion Hotel B66 Baskin-Robbins D36 The Albion Hotel B60 Battlezone Playlive E91 Alice’s Wonderland Fancy Dress Hire G65 Baume A77 Al-Oi Thai Restaurant A66 Bay of India C37 Alpine Sports G69 The Bayview B56 Amandas on the Edge A9 BCF F19, 20 Amazement E69 Beach Hotel B13 The Anchor B68 The Beehive Honeysuckle B96 And the Winner Is OSCARS B53 Bella Beans B97 Apollo Motorhome Holidays H65, 66 The Belmore Hotel B52 Aqua Golf E24 The Bikesmith & Espresso Bar D61 Aqua re Bar & Grill B67 Bimbadgen G18 Arabian Lounge C25 Birdy’s Refreshments and Espresso B125 Arajilla J15, 16 Black Circle Cafe B95 The Argenton Hotel B27 Black Pepper Butchery G5 The Ark Cafe B69 Aromas on Sea B16 Blackbird Artisan Bakery B104 Art Series Hotel Group J39, 40 The Blackbutt Hotel B76 Astral Tower J11, 12 Blaxland Inn A65 The Australia Hotel B24 Bliss Coffee Roasters D66 Australia Walkabout Wildlife Park E87, 88 Blue and White Car Wash G82 Australia Zoo H29, 30 Bluebird Florist G76 Australian Boating College E65 Bocados Spanish Kitchen A50 Australian Outback Spectacular H27, 28 Bolton Street Pantry B47 Australian Reptile Park E3 Bondi Pizza - Bar & Grill A85 Visit www.entertainmentbook.com.au for additional offers, suburb search, important updates and more. -

Incursions/ Excursions
CMA Region Examples of Incursions/ Excursions (location) Marine and Freshwater Discovery Centre – Queenscliff Corangamite Narana Aboriginal Cultural Centre – Geelong Conservation Ecology Centre – Cape Otway Black Snake Company East Gippsland Fishcare Bug Blitz Meet the maremmas – penguin guard dogs tours at Warrnambool. Glenelg Hopkins Budj Bim tours of World Heritage listed National Heritage Landscapes at Lake Condah. Tour of Yatmerone Reserve through the Penshurst Volcanoes Discovery Centre. Winton Wetlands Euroa Arboretum Goulburn Broken Mansfield Zoo Shepparton Botanic Gardens Yea Wetlands Welcome to the Kyabram Fauna Park - Protecting Australia's Wildlife Heritage Mallee Environmental Education at the Mildura Eco Village Strathallan Landcare Group- Squirrel Glider Sanctuary Tours (Echuca area) North Central PepperGreen Farm (Bendigo Based) TZR Reptiles and Wildlife Incursions (Bendigo Based) Wild Action Zoo (Macedon based) North East SEED School Excursions & Educational Directory Friends of the Mitta Melbourne’s Living Museum of the West CERES Edithvale-Seaford Wetland Education Centre Ecolink Healesville Sanctuary Port Phillip & Mt Rothwell Western Port Phillip Island Nature Parks Port Phillip Eco Centre Marine and Freshwater Discovery Centre Royal Botanic Gardens Victoria- Melbourne and Cranbourne Serendip Sanctuary Waterwatch Bunurong Environment Centre, Inverloch West Gippsland Bass Coast’s Environmental Detectives Heart Morass with Bug Blitz Trust Little Desert Nature Lodge Wimmera Halls Gap Zoo Jamie & Kims Mobile Zoo Wildlife incursions 2021 Victorian Junior Landcare and Biodiversity Grants – Incursions/Excursions www.landcareaustralia.org.au/victorian-junior-landcare-biodiversity-grants 2021 Victorian Junior Landcare and Biodiversity Grants – Incursions/Excursions www.landcareaustralia.org.au/victorian-junior-landcare-biodiversity-grants . -

Emergency Response to Australia's Black Summer 2019–2020
animals Commentary Emergency Response to Australia’s Black Summer 2019–2020: The Role of a Zoo-Based Conservation Organisation in Wildlife Triage, Rescue, and Resilience for the Future Marissa L. Parrott 1,*, Leanne V. Wicker 1,2, Amanda Lamont 1, Chris Banks 1, Michelle Lang 3, Michael Lynch 4, Bonnie McMeekin 5, Kimberly A. Miller 2, Fiona Ryan 1, Katherine E. Selwood 1, Sally L. Sherwen 1 and Craig Whiteford 1 1 Wildlife Conservation and Science, Zoos Victoria, Parkville, VIC 3052, Australia; [email protected] (L.V.W.); [email protected] (A.L.); [email protected] (C.B.); [email protected] (F.R.); [email protected] (K.E.S.); [email protected] (S.L.S.); [email protected] (C.W.) 2 Healesville Sanctuary, Badger Creek, VIC 3777, Australia; [email protected] 3 Marketing, Communications & Digital Strategy, Zoos Victoria, Parkville, VIC 3052, Australia; [email protected] 4 Melbourne Zoo, Parkville, VIC 3052, Australia; [email protected] 5 Werribee Open Range Zoo, Werribee, VIC 3030, Australia; [email protected] * Correspondence: [email protected] Simple Summary: In the summer of 2019–2020, a series of more than 15,000 bushfires raged across Citation: Parrott, M.L.; Wicker, L.V.; Australia in a catastrophic event called Australia’s Black Summer. An estimated 3 billion native Lamont, A.; Banks, C.; Lang, M.; animals, and whole ecosystems, were impacted by the bushfires, with many endangered species Lynch, M.; McMeekin, B.; Miller, K.A.; pushed closer to extinction. Zoos Victoria was part of a state-led bushfire response to assist wildlife, Ryan, F.; Selwood, K.E.; et al. -

Download the Annual Report 2019-2020
Leading � rec�very Annual Report 2019–2020 TARONGA ANNUAL REPORT 2019–2020 A SHARED FUTURE � WILDLIFE AND PE�PLE At Taronga we believe that together we can find a better and more sustainable way for wildlife and people to share this planet. Taronga recognises that the planet’s biodiversity and ecosystems are the life support systems for our own species' health and prosperity. At no time in history has this been more evident, with drought, bushfires, climate change, global pandemics, habitat destruction, ocean acidification and many other crises threatening natural systems and our own future. Whilst we cannot tackle these challenges alone, Taronga is acting now and working to save species, sustain robust ecosystems, provide experiences and create learning opportunities so that we act together. We believe that all of us have a responsibility to protect the world’s precious wildlife, not just for us in our lifetimes, but for generations into the future. Our Zoos create experiences that delight and inspire lasting connections between people and wildlife. We aim to create conservation advocates that value wildlife, speak up for nature and take action to help create a future where both people and wildlife thrive. Our conservation breeding programs for threatened and priority wildlife help a myriad of species, with our program for 11 Legacy Species representing an increased commitment to six Australian and five Sumatran species at risk of extinction. The Koala was added as an 11th Legacy Species in 2019, to reflect increasing threats to its survival. In the last 12 months alone, Taronga partnered with 28 organisations working on the front line of conservation across 17 countries. -

The Oldest Platypus and Its Bearing on Divergence Timing of the Platypus and Echidna Clades
The oldest platypus and its bearing on divergence timing of the platypus and echidna clades Timothy Rowe*†, Thomas H. Rich‡§, Patricia Vickers-Rich§, Mark Springer¶, and Michael O. Woodburneʈ *Jackson School of Geosciences, University of Texas, C1100, Austin, TX 78712; ‡Museum Victoria, PO Box 666, Melbourne, Victoria 3001, Australia; §School of Geosciences, PO Box 28E, Monash University, Victoria 3800, Australia; ¶Department of Biology, University of California, Riverside, CA 92521; and ʈDepartment of Geology, Museum of Northern Arizona, Flagstaff, AZ 86001 Edited by David B. Wake, University of California, Berkeley, CA, and approved October 31, 2007 (received for review July 7, 2007) Monotremes have left a poor fossil record, and paleontology has broadly affect our understanding of early mammalian history, been virtually mute during two decades of discussion about with special implications for molecular clock estimates of basal molecular clock estimates of the timing of divergence between the divergence times. platypus and echidna clades. We describe evidence from high- Monotremata today comprises five species that form two resolution x-ray computed tomography indicating that Teinolo- distinct clades (16). The echidna clade includes one short-beaked phos, an Early Cretaceous fossil from Australia’s Flat Rocks locality species (Tachyglossus aculeatus; Australia and surrounding is- (121–112.5 Ma), lies within the crown clade Monotremata, as a lands) and three long-beaked species (Zaglossus bruijni, Z. basal platypus. Strict molecular clock estimates of the divergence bartoni, and Z. attenboroughi, all from New Guinea). The between platypus and echidnas range from 17 to 80 Ma, but platypus clade includes only Ornithorhynchus anatinus (Austra- Teinolophos suggests that the two monotreme clades were al- lia, Tasmania). -

Draft Brisbane Botanic Gardens Mt Coot-Tha Master Plan 2017 Sets the Vision and Strategic Framework to Guide the Next Generation of Growth in the Gardens
Brisbane Botanic Gardens MT COOT-THA MT COOT-THA DRAFT A message from Lord Mayor Graham Quirk As Lord Mayor of Brisbane, I am focussed on ensuring the lifestyle, sustainability and liveability of our city is preserved and enhanced. Brisbane’s green spaces are an important part of our identity and play a significant role in making Brisbane City one of the best places to live, work and play. Recognised as Queensland’s premier subtropical botanic gardens, and spanning over 56 hectares, Brisbane Botanic Gardens Mt Coot-tha offers unique lifestyle opportunities for residents and visitors. Visitor numbers to the Gardens are anticipated to increase from 700,000 to more than 1.7 million visitors per year in the next 15 years and this draft master plan focuses on the key opportunities and future direction for the Gardens during this time. I encourage you to have your say and help us shape the future of this iconic green landmark for the City of Brisbane. Contents 1 Introduction page 3 2 Strategic Context page 4 3 Opportunities and Challenges page 8 4 Vision page 10 5 Themes page 12 6 The Master Plan page 14 Site-wide Ideas Entry Precinct Lakeside Precinct Central Precinct Retreat Precinct Mt Coot-tha Precinct 7 A living garden page 30 2 brisbane botanic gardens mt coot-tha INTRODUCTION The Brisbane Botanic Gardens Mt Coot-tha was opened by Brisbane City Council in 1976. It has grown to become Australia’s largest subtropical botanic gardens, featuring more than 200,000 plants that represent approximately 5000 species from around the world. -
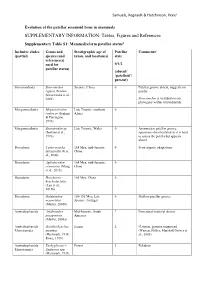
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus -

Bird Places of Brisbane
Brisbane’s Birds. Brisbane is home to around 400 species of birds. Some are here for only part of the year. The birds are here because of our sub-tropical climate and the variety Birds Queensland is a non-profit organization, which promotes the Bird of habitats which include beaches, mud flat, sand bank, foreshore, appreciation, conservation and scientific study of birds. mangrove, salt marsh, bushland, urban park, playing field and the extensive vegetation in thousands of private gardens. Activities include; • Monthly meetings with interesting speakers. Places Walking in any park, along a track, a bikeway, an urban street, a bush • Bird identification classes. land area or beside a creek will reveal a surprising number of birds. • Walks and camps to see and enjoy birds. • Raising funds for scientific bird research. of Pressure for housing and development is causing bird habitat to • Publishing a monthly newsletter and a scientific journal. Brisbane disappear. Small bush birds are particularly vulnerable. • Collecting data on bird distribution. Where to find native birds in Brisbane • Working with local authorities to conserve habitat. Brisbane City Council is actively retaining natural areas, rehabilitating degraded ones and maintaining native vegetation along creeks and These activities are open to all members. corridors. Non-members are also welcome. Watching birds is an enjoyable and rewarding Birds Queensland meets pastime. Binoculars make birdwatching easier. on the first Thursday of each month (except January) starting 7:40 pm at the Birds Queensland’s brochure Royal Geographical Society Building “What’s That Bird?” 237 Milton Road, Milton has a short bird list with illustrations. -

Australia's Wildlife Ark Annual Report
AUSTRALIA’S WILDLIFE ARK ANNUAL REPORT 2017 – 2018 Financial Year ABN: 51 417 871 203 VISION Creating a long-term future for Australia’s threatened wildlife. MISSION • To protect Australia’s threatened species with robust insurance populations, • To create healthy ecosystems within Aussie Ark sanctuaries and through rewilding, • To have long-term tangible outcomes for the species in our care, • To be a proactive, professional, transparent, and effective organisation. Every effort has been made to ensure the accuracy of the 2017-2018 Australia’s Wildlife Ark Incorporated Annual Report. We apologise if any omissions or errors have occurred. If you discover an error or omission, please notify the Secretary & Public Officer, Liz Gabriel at [email protected] TABLE OF CONTENTS Committee of Management 4 President’s Report 5 Director’s Report 6 Project Partners 7 Key Achievements 8 Financial Report 9 Conservation and Operations 26 Human Resources Living Collection Tertiary Education groups Facility Operations Marketing and Communications 30 Website Social Media Email Marketing Public Relations 32 Media Community Relations – Aussie Ark Advocates Fundraising and Administration 37 Income Successful Grants Corporate Donors and Sponsorships Gifts in Kind Acquisition Administration and Expenditure 3 COMMITTEE OF MANAGEMENT COMMITTEE OF MANAGEMENT Tim Faulkner – President Bruce Kubbere – Vice President Liz Gabriel – Treasurer & Secretary Chris Chapman – Committee of Management Member Paul Andrew – Committee of Management Member Brad Walker – Committee -

Constituents of Platypus and Echidna Milk, with Particular Reference to the Fatty Acid Complement of the Triglycerides
Aust. J. Bioi. Sci., 1984, 37, 323-9 Constituents of Platypus and Echidna Milk, with Particular Reference to the Fatty Acid Complement of the Triglycerides Mervyn Grijfiths,A,B Brian Green,A R. M. C. Leckie,A Michael Messerc and K. W. NewgrainA A Division of Wildlife and Rangelands Research, CSIRO, P.O. Box 84, Lyneham, A.C.T. 2602. B Department of Physical Biochemistry, Australian National University, G.P.O. Box 334, Canberra, A.C.T. 2601. cDepartment of Biochemistry, University of Sydney, Sydney, N.S.W. 2006. Abstract The mature milk of platypuses (Ornithorhynchus anatinus) exhibit 39· 1 gf 100 g solids, of which crude lipid accounts for 22·2% crude protein 8·2%, hexose 3·3% and sialic acid 0·43%; iron is in high concentration, 21·1 mgfl. Echidna (Tachyglossus aculeatus) milk is more concentrated, containing 48·9% solids, with lipid. protein, hexose and sialic acid accounting for 31 ·0, 12·4, 1· 6 and O· 70% respectively; it is even richer in iron, 33·3 mgfl. The fatty acid complement of milk triglyceride from platypuses living in their natural habitat is different from that of the echidna living in the wild: platypus milk triglyceride contains 17·6% palmitic, 25 . 2% oleic and c. 30% long-chain polyunsaturated acids, whereas that of echidnas exhibits 15· 9% palmitic, 61 ·2% oleic and only c. 7% polyunsaturated acids. The fatty acid complement of echidna milk triglyceride can be changed by altering the fatty acid complement of the dietary lipid. Introduction Levels of the proximate constituents, i.e. total solids, crude lipid, crude protein, carbohydrates and minerals, were determined in samples of platypus and echidna milk.