Description of a Cranial Endocast from a Fossil Platypus, Obdurodon Dicksoni
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Beyond Endocasts: Using Predicted Brain-Structure Volumes of Extinct Birds to Assess Neuroanatomical and Behavioral Inferences
diversity Article Beyond Endocasts: Using Predicted Brain-Structure Volumes of Extinct Birds to Assess Neuroanatomical and Behavioral Inferences 1, , 2 2 Catherine M. Early * y , Ryan C. Ridgely and Lawrence M. Witmer 1 Department of Biological Sciences, Ohio University, Athens, OH 45701, USA 2 Department of Biomedical Sciences, Heritage College of Osteopathic Medicine, Ohio University, Athens, OH 45701, USA; [email protected] (R.C.R.); [email protected] (L.M.W.) * Correspondence: [email protected] Current Address: Florida Museum of Natural History, University of Florida, Gainesville, FL 32611, USA. y Received: 1 November 2019; Accepted: 30 December 2019; Published: 17 January 2020 Abstract: The shape of the brain influences skull morphology in birds, and both traits are driven by phylogenetic and functional constraints. Studies on avian cranial and neuroanatomical evolution are strengthened by data on extinct birds, but complete, 3D-preserved vertebrate brains are not known from the fossil record, so brain endocasts often serve as proxies. Recent work on extant birds shows that the Wulst and optic lobe faithfully represent the size of their underlying brain structures, both of which are involved in avian visual pathways. The endocasts of seven extinct birds were generated from microCT scans of their skulls to add to an existing sample of endocasts of extant birds, and the surface areas of their Wulsts and optic lobes were measured. A phylogenetic prediction method based on Bayesian inference was used to calculate the volumes of the brain structures of these extinct birds based on the surface areas of their overlying endocast structures. This analysis resulted in hyperpallium volumes of five of these extinct birds and optic tectum volumes of all seven extinct birds. -

Miocene Mammal Reveals a Mesozoic Ghost Lineage on Insular New Zealand, Southwest Pacific
Miocene mammal reveals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific Trevor H. Worthy*†, Alan J. D. Tennyson‡, Michael Archer§, Anne M. Musser¶, Suzanne J. Hand§, Craig Jonesʈ, Barry J. Douglas**, James A. McNamara††, and Robin M. D. Beck§ *School of Earth and Environmental Sciences, Darling Building DP 418, Adelaide University, North Terrace, Adelaide 5005, South Australia, Australia; ‡Museum of New Zealand Te Papa Tongarewa, P.O. Box 467, Wellington 6015, New Zealand; §School of Biological, Earth and Environmental Sciences, University of New South Wales, New South Wales 2052, Australia; ¶Australian Museum, 6-8 College Street, Sydney, New South Wales 2010, Australia; ʈInstitute of Geological and Nuclear Sciences, P.O. Box 30368, Lower Hutt 5040, New Zealand; **Douglas Geological Consultants, 14 Jubilee Street, Dunedin 9011, New Zealand; and ††South Australian Museum, Adelaide, South Australia 5000, Australia Edited by James P. Kennett, University of California, Santa Barbara, CA, and approved October 11, 2006 (sent for review July 8, 2006) New Zealand (NZ) has long been upheld as the archetypical Ma) dinosaur material (13) and isolated moa bones from marine example of a land where the biota evolved without nonvolant sediments up to 2.5 Ma (1, 14), the terrestrial record older than terrestrial mammals. Their absence before human arrival is mys- 1 Ma is extremely limited. Until now, there has been no direct terious, because NZ was still attached to East Antarctica in the Early evidence for the pre-Pleistocene presence in NZ of any of its Cretaceous when a variety of terrestrial mammals occupied the endemic vertebrate lineages, particularly any group of terrestrial adjacent Australian portion of Gondwana. -

The Oldest Platypus and Its Bearing on Divergence Timing of the Platypus and Echidna Clades
The oldest platypus and its bearing on divergence timing of the platypus and echidna clades Timothy Rowe*†, Thomas H. Rich‡§, Patricia Vickers-Rich§, Mark Springer¶, and Michael O. Woodburneʈ *Jackson School of Geosciences, University of Texas, C1100, Austin, TX 78712; ‡Museum Victoria, PO Box 666, Melbourne, Victoria 3001, Australia; §School of Geosciences, PO Box 28E, Monash University, Victoria 3800, Australia; ¶Department of Biology, University of California, Riverside, CA 92521; and ʈDepartment of Geology, Museum of Northern Arizona, Flagstaff, AZ 86001 Edited by David B. Wake, University of California, Berkeley, CA, and approved October 31, 2007 (received for review July 7, 2007) Monotremes have left a poor fossil record, and paleontology has broadly affect our understanding of early mammalian history, been virtually mute during two decades of discussion about with special implications for molecular clock estimates of basal molecular clock estimates of the timing of divergence between the divergence times. platypus and echidna clades. We describe evidence from high- Monotremata today comprises five species that form two resolution x-ray computed tomography indicating that Teinolo- distinct clades (16). The echidna clade includes one short-beaked phos, an Early Cretaceous fossil from Australia’s Flat Rocks locality species (Tachyglossus aculeatus; Australia and surrounding is- (121–112.5 Ma), lies within the crown clade Monotremata, as a lands) and three long-beaked species (Zaglossus bruijni, Z. basal platypus. Strict molecular clock estimates of the divergence bartoni, and Z. attenboroughi, all from New Guinea). The between platypus and echidnas range from 17 to 80 Ma, but platypus clade includes only Ornithorhynchus anatinus (Austra- Teinolophos suggests that the two monotreme clades were al- lia, Tasmania). -

EDITORIAL NOTE Collection of Paleontology Papers in Honor of The
Anais da Academia Brasileira de Ciências (2019) 91(Suppl. 2): e20191434 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 http://dx.doi.org/10.1590/0001-3765201920191434 www.scielo.br/aabc | www.fb.com/aabcjournal EDITORIAL NOTE Collection of Paleontology Papers in honor of the Centenary of the Brazilian Academy of Sciences ALEXANDER W.A. KELLNER* and MARINA B. SOARES Laboratório de Sistemática e Tafonomia de Vertebrados Fósseis, Departamento de Geologia e Paleontologia do Museu Nacional/UFRJ, Quinta da Boa Vista, s/n, São Cristóvão, 20940-040 Rio de Janeiro, RJ, Brazil How to cite: KELLNER AWA AND SOARES MB. 2019. Collection of Paleontology Papers in honor of the Centenary of the Brazilian Academy of Sciences. An Acad Bras Cienc 91: e20191434. DOI 10.1590/0001-3765201920191434. The Brazilian Academy of Sciences is a non-profit organization (ABC 2019) that has completed one century of existence in 2016. A series of special publications was organized by the Annals of the Brazilian Academy of Sciences (AABC) in celebration of this important date (e.g., Kellner 2017, Crespilho 2018, Cavaleiro 2018). Here we have the pleasure to introduce the final of these volumes gathering 20 original contributions in paleontology, the science dedicated to the study of all evidences of life that have been preserved in layers of deep time. The topics presented here vary from the description of new species and specimens of flying reptiles, dinosaurs, and crocodylomorphs to studies on biogeography, osteohistology, and specific contributions provided by microfossils. Over 70 authors from different countries were involved in this volume, showing the increasing international integration of Brazilian paleontologists. -

On the Paleontology of Animal Cognition: Using the Brain Dimensions of Modern Birds to Characterize Maniraptor Cognition
12 Journal of Advanced Neuroscience Research, Special Issue, May-2017, 12-19 On the Paleontology of Animal Cognition: Using the Brain Dimensions of Modern Birds to Characterize Maniraptor Cognition Thomas M. Gaetano1, Margaret M. Yacobucci1 and Verner P. Bingman2,* 1Department of Geology, Bowling Green State University, Ohio, USA 2Department of Psychology and J.P. Scott Center for Neuroscience, Mind and Behavior, Bowling Green State University, Ohio, USA Abstract: Drawing inferences on the characteristics, including behavior, of extinct species using comparisons with extant species has a long tradition in paleontology. Departing from the observation that extinct maniraptors possessed brains with a relatively long and narrow telencephalon, we used digital endocasts taken from 11 species of modern birds to determine if any of the sampled modern bird species displayed a similar telencephalic shape, and by inference, similar cognitive ability. The analysis revealed that the telencephalon of the double-crested cormorant (Phalacrocorax auritus) is extraordinarily narrow (large length-to-width ratio) and strikingly similar to Archaeopteryx and even some non-avian, maniraptoran dinosaurs. The relatively narrow brain in turn suggests a relatively small nidopallium subdivision of the telencephalon and associated impoverished general cognitive ability. This first-order brain-anatomical observation, together with the relatively ancient origins of a cormorant fossil record, suggest that cormorants could be used as a model for the general cognitive abilities of extinct maniraptors. Keywords: Brain endocasts, Comparative cognition, Double-crested cormorant, Hippocampus, Nidopallium, Paleoneurology. INTRODUCTION animal cognition, observations and experimental evidence from birds have also offered some extraordi- Considerations of the richness of animal cognitive nary examples of cognition [3]. -

Homo Erectus: a Bigger, Faster, Smarter, Longer Lasting Hominin Lineage
Homo erectus: A Bigger, Faster, Smarter, Longer Lasting Hominin Lineage Charles J. Vella, PhD August, 2019 Acknowledgements Many drawings by Kathryn Cruz-Uribe in Human Career, by R. Klein Many graphics from multiple journal articles (i.e. Nature, Science, PNAS) Ray Troll • Hominin evolution from 3.0 to 1.5 Ma. (Species) • Currently known species temporal ranges for Pa, Paranthropus aethiopicus; Pb, P. boisei; Pr, P. robustus; A afr, Australopithecus africanus; Ag, A. garhi; As, A. sediba; H sp., early Homo >2.1 million years ago (Ma); 1470 group and 1813 group representing a new interpretation of the traditionally recognized H. habilis and H. rudolfensis; and He, H. erectus. He (D) indicates H. erectus from Dmanisi. • (Behavior) Icons indicate from the bottom the • first appearance of stone tools (the Oldowan technology) at ~2.6 Ma, • the dispersal of Homo to Eurasia at ~1.85 Ma, • and the appearance of the Acheulean technology at ~1.76 Ma. • The number of contemporaneous hominin taxa during this period reflects different Susan C. Antón, Richard Potts, Leslie C. Aiello, 2014 strategies of adaptation to habitat variability. Origins of Homo: Summary of shifts in Homo Early Homo appears in the record by 2.3 Ma. By 2.0 Ma at least two facial morphs of early Homo (1813 group and 1470 group) representing two different adaptations are present. And possibly 3 others as well (Ledi-Geraru, Uraha-501, KNM-ER 62000) The 1813 group survives until at least 1.44 Ma. Early Homo erectus represents a third more derived morph and one that is of slightly larger brain and body size but somewhat smaller tooth size. -

1000002291104.Pdf
Quaternary International 211 (2010) 4–13 Contents lists available at ScienceDirect Quaternary International journal homepage: www.elsevier.com/locate/quaint Morphological and morphometric analysis of variation in the Zhoukoudian Homo erectus brain endocasts Xiujie Wu a,b,*, Lynne A. Schepartz c, Christopher J. Norton d a Laboratory of Evolutionary Systematics of Vertebrates, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Xizhimenwaidajie 142, Box 643, Beijing 100044, China b State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Nanjing 210008, China c Department of Anthropology, Florida State University, Tallahassee, FL 32306-7772, USA d Department of Anthropology, University of Hawaii at Manoa, Honolulu, HI 96822-2223, USA article info abstract Article history: The six Zhoukoudian (ZKD) Locality 1 Homo erectus specimens derive from stratigraphic levels 11–3 with Available online 19 July 2009 a geochronological span of approximately 0.3 Ma. This paper introduces the history of the ZKD endocasts and presents data on their morphological features and linear dimensions in order to evaluate variability in the sample over time and in the broader context of human brain evolution using a comparative sample of African and other Asian H. erectus fossils and modern Chinese males. The ZKD brains are very similar in their morphological characteristics, but there are also significant but subtle changes involving expansion of the frontal and occipital lobe breadths that correlate with the geochronology. The same is not true for general endocranial volume. The ZKD brains, together with other Asian and African H. erectus specimens, have low height dimensions and short parietal chords that distinguish them from the modern Chinese. -

Ontogenetic and Adult Shape Variation in the Endocast of Tapirus: Implications for T
East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations Student Works 5-2020 Ontogenetic and Adult Shape Variation in the Endocast of Tapirus: Implications for T. polkensis from the Gray Fossil Site Thomas M. Gaetano East Tennessee State University Follow this and additional works at: https://dc.etsu.edu/etd Part of the Other Neuroscience and Neurobiology Commons, Paleobiology Commons, Paleontology Commons, and the Zoology Commons Recommended Citation Gaetano, Thomas M., "Ontogenetic and Adult Shape Variation in the Endocast of Tapirus: Implications for T. polkensis from the Gray Fossil Site" (2020). Electronic Theses and Dissertations. Paper 3765. https://dc.etsu.edu/etd/3765 This Thesis - unrestricted is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. Ontogenetic and Adult Shape Variation in the Endocast of Tapirus: Implications for T. polkensis from the Gray Fossil Site ________________________________ A thesis presented to the faculty of the Department of Geosciences East Tennessee State University In partial fulfillment of the requirements for the degree Masters of Science in Geosciences _________________________________ by Thomas M. Gaetano May 2020 _________________________________ Dr. Steven C. Wallace, Chair Dr. Christopher C. Widga Dr. Richard T. Carter Dr. Thomas C. Jones Keywords: Endocast, Paleoneurology, Tapir, Gray Fossil Site, Telencephalon, Sociality, Paleoecology, Sensory Ecology ABSTRACT Ontogenetic and Adult Shape Variation in the Endocast of Tapirus: Implications for T. -

Early Cretaceous Amphilestid ('Triconodont') Mammals from Mongolia
Early Cretaceous amphilestid ('triconodont') mammals from Mongolia ZOFIAKIELAN-JAWOROWSKA and DEMBERLYIN DASHZEVEG Kielan-Jaworowską Z. &Daslueveg, D. 1998. Early Cretaceous amphilestid (.tricono- dont') mammals from Mongotia. - Acta Pal.aeontol.ogicaPolonica,43,3, 413438. Asmall collection of ?Aptianor ?Albian amphilestid('triconodont') mammals consisting of incomplete dentaries and maxillae with teeth, from the Khoboor localiĘ Guchin Us counĘ in Mongolia, is described. Grchinodon Troftmov' 1978 is regarded a junior subjective synonym of GobiconodonTroftmov, 1978. Heavier wear of the molariforms M3 andM4than of themore anteriorone-M2 in Gobiconodonborissiaki gives indirect evidence formolariformreplacement in this taxon. The interlocking mechanismbetween lower molariforms n Gobiconodon is of the pattern seen in Kuchneotherium and Ttnodon. The ińterlocking mechanism and the type of occlusion ally Amphilestidae with Kuehneotheriidae, from which they differ in having lower molariforms with main cusps aligned and the dentary-squamosal jaw joint (double jaw joint in Kuehneotheńdae). The main cusps in upper molariforms M3-M5 of Gobiconodon, however, show incipient tńangular arrangement. The paper gives some support to Mills' idea on the therian affinities of the Amphilestidae, although it cannot be excluded that the characters that unite the two groups developed in parallel. Because of scanty material and arnbiguĘ we assign the Amphilestidae to order incertae sedis. Key words : Mammali4 .triconodonts', Amphilestidae, Kuehneotheriidae, Early Cretaceous, Mongolia. Zofia Kiel,an-Jaworowska [zkielnn@twarda,pan.pl], InsĘtut Paleobiologii PAN, ul. Twarda 5 I /5 5, PL-00-8 I 8 Warszawa, Poland. DemberĘin Dash7eveg, Geological Institute, Mongolian Academy of Sciences, Ulan Bator, Mongolia. Introduction Beliajeva et al. (1974) reportedthe discovery of Early Cretaceous mammals at the Khoboor locality (referred to also sometimes as Khovboor), in the Guchin Us Soinon (County), Gobi Desert, Mongolia. -
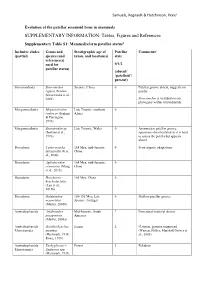
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus -

New Australopithecine Endocast, SK 1585, from Swartkrans, South Africa
New Australopithecine Endocast, SK 1585, from Swartkrans, South Africa RALPH L. HOLLOWAY Deprrrtmrnt of Anthropology, Colttinbin University, New York, New York 10027 KEY WORDS Brain . Primates . Endocasts . Australopithecines. ABSTRACT The new SK 1585 endocast, found by Dr. Brain at Swartkrans, 1966, is that of a robust australopithecine, matching the endocast of the Olduvai Hominid 5 in volume, and being almost identical to it in morphology. Aside from Olduvai Hominid 5 it is the only robust australopithecine endocast com- plete enough to permit easy reconstruction, as only a small portion of the frontal lobe is missing. While the gyral and sulcal patterns are not clear, there are a number of features indicating that the brain is not that of a pongid, but that is has been reorganized to a hominid pattern, particularly the occipital, parietal, and temporal lobes. A new undistorted endocast, presuma- of visual inspection, after the surface of bly that of a robust australopithecine, the cast had been lightly rubbed with was discovered in January, 1966, by carbon shavings to enhance the surface Dr. C. K. Brain in the hillside dump rub- detail. Various combinations of lighting ble at Swartkrans (Brain, '70, '67). At the were employed to bring out features of kmd suggestion of Dr. Brain, I was in- cerebral morphology. vited to describe this endocast while visit- The volumetric determinations by water ing in Johannesburg during the early part displacement were carried out on plasti- of 1969. I am greatly indebted to Dr. cine reconstructed plaster copy, first Brain and Prof. P. V. Tobias for this checked for its accuracy against the orig- opportunity. -

Constituents of Platypus and Echidna Milk, with Particular Reference to the Fatty Acid Complement of the Triglycerides
Aust. J. Bioi. Sci., 1984, 37, 323-9 Constituents of Platypus and Echidna Milk, with Particular Reference to the Fatty Acid Complement of the Triglycerides Mervyn Grijfiths,A,B Brian Green,A R. M. C. Leckie,A Michael Messerc and K. W. NewgrainA A Division of Wildlife and Rangelands Research, CSIRO, P.O. Box 84, Lyneham, A.C.T. 2602. B Department of Physical Biochemistry, Australian National University, G.P.O. Box 334, Canberra, A.C.T. 2601. cDepartment of Biochemistry, University of Sydney, Sydney, N.S.W. 2006. Abstract The mature milk of platypuses (Ornithorhynchus anatinus) exhibit 39· 1 gf 100 g solids, of which crude lipid accounts for 22·2% crude protein 8·2%, hexose 3·3% and sialic acid 0·43%; iron is in high concentration, 21·1 mgfl. Echidna (Tachyglossus aculeatus) milk is more concentrated, containing 48·9% solids, with lipid. protein, hexose and sialic acid accounting for 31 ·0, 12·4, 1· 6 and O· 70% respectively; it is even richer in iron, 33·3 mgfl. The fatty acid complement of milk triglyceride from platypuses living in their natural habitat is different from that of the echidna living in the wild: platypus milk triglyceride contains 17·6% palmitic, 25 . 2% oleic and c. 30% long-chain polyunsaturated acids, whereas that of echidnas exhibits 15· 9% palmitic, 61 ·2% oleic and only c. 7% polyunsaturated acids. The fatty acid complement of echidna milk triglyceride can be changed by altering the fatty acid complement of the dietary lipid. Introduction Levels of the proximate constituents, i.e. total solids, crude lipid, crude protein, carbohydrates and minerals, were determined in samples of platypus and echidna milk.