Conservation of Energy External Forces
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
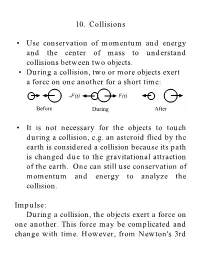
10. Collisions • Use Conservation of Momentum and Energy and The
10. Collisions • Use conservation of momentum and energy and the center of mass to understand collisions between two objects. • During a collision, two or more objects exert a force on one another for a short time: -F(t) F(t) Before During After • It is not necessary for the objects to touch during a collision, e.g. an asteroid flied by the earth is considered a collision because its path is changed due to the gravitational attraction of the earth. One can still use conservation of momentum and energy to analyze the collision. Impulse: During a collision, the objects exert a force on one another. This force may be complicated and change with time. However, from Newton's 3rd Law, the two objects must exert an equal and opposite force on one another. F(t) t ti tf Dt From Newton'sr 2nd Law: dp r = F (t) dt r r dp = F (t)dt r r r r tf p f - pi = Dp = ò F (t)dt ti The change in the momentum is defined as the impulse of the collision. • Impulse is a vector quantity. Impulse-Linear Momentum Theorem: In a collision, the impulse on an object is equal to the change in momentum: r r J = Dp Conservation of Linear Momentum: In a system of two or more particles that are colliding, the forces that these objects exert on one another are internal forces. These internal forces cannot change the momentum of the system. Only an external force can change the momentum. The linear momentum of a closed isolated system is conserved during a collision of objects within the system. -
![Arxiv:1902.03186V3 [Math.AP]](https://docslib.b-cdn.net/cover/7314/arxiv-1902-03186v3-math-ap-157314.webp)
Arxiv:1902.03186V3 [Math.AP]
PRIMITIVE EQUATIONS WITH HORIZONTAL VISCOSITY: THE INITIAL VALUE AND THE TIME-PERIODIC PROBLEM FOR PHYSICAL BOUNDARY CONDITIONS AMRU HUSSEIN, MARTIN SAAL, AND MARC WRONA Abstract. The 3D-primitive equations with only horizontal viscosity are con- sidered on a cylindrical domain Ω = (−h,h) × G, G ⊂ R2 smooth, with the physical Dirichlet boundary conditions on the sides. Instead of considering a vanishing vertical viscosity limit, we apply a direct approach which in particu- lar avoids unnecessary boundary conditions on top and bottom. For the initial value problem, we obtain existence and uniqueness of local z-weak solutions for initial data in H1((−h,h),L2(G)) and local strong solutions for initial data 1 1 2 q in H (Ω). If v0 ∈ H ((−h,h),L (G)), ∂zv0 ∈ L (Ω) for q > 2, then the z- weak solution regularizes instantaneously and thus extends to a global strong solution. This goes beyond the global well-posedness result by Cao, Li and Titi (J. Func. Anal. 272(11): 4606-4641, 2017) for initial data near H1 in the periodic setting. For the time-periodic problem, existence and uniqueness of z-weak and strong time periodic solutions is proven for small forces. Since this is a model with hyperbolic and parabolic features for which classical results are not directly applicable, such results for the time-periodic problem even for small forces are not self-evident. 1. Introduction and main results The 3D-primitive equations are one of the fundamental models for geophysical flows, and they are used for describing oceanic and atmospheric dynamics. They are derived from the Navier-Stokes equations assuming a hydrostatic balance. -

Lecture 18 Ocean General Circulation Modeling
Lecture 18 Ocean General Circulation Modeling 9.1 The equations of motion: Navier-Stokes The governing equations for a real fluid are the Navier-Stokes equations (con servation of linear momentum and mass mass) along with conservation of salt, conservation of heat (the first law of thermodynamics) and an equation of state. However, these equations support fast acoustic modes and involve nonlinearities in many terms that makes solving them both difficult and ex pensive and particularly ill suited for long time scale calculations. Instead we make a series of approximations to simplify the Navier-Stokes equations to yield the “primitive equations” which are the basis of most general circu lations models. In a rotating frame of reference and in the absence of sources and sinks of mass or salt the Navier-Stokes equations are @ �~v + �~v~v + 2�~ �~v + g�kˆ + p = ~ρ (9.1) t r · ^ r r · @ � + �~v = 0 (9.2) t r · @ �S + �S~v = 0 (9.3) t r · 1 @t �ζ + �ζ~v = ω (9.4) r · cpS r · F � = �(ζ; S; p) (9.5) Where � is the fluid density, ~v is the velocity, p is the pressure, S is the salinity and ζ is the potential temperature which add up to seven dependent variables. 115 12.950 Atmospheric and Oceanic Modeling, Spring '04 116 The constants are �~ the rotation vector of the sphere, g the gravitational acceleration and cp the specific heat capacity at constant pressure. ~ρ is the stress tensor and ω are non-advective heat fluxes (such as heat exchange across the sea-surface).F 9.2 Acoustic modes Notice that there is no prognostic equation for pressure, p, but there are two equations for density, �; one prognostic and one diagnostic. -

Law of Conversation of Energy
Law of Conservation of Mass: "In any kind of physical or chemical process, mass is neither created nor destroyed - the mass before the process equals the mass after the process." - the total mass of the system does not change, the total mass of the products of a chemical reaction is always the same as the total mass of the original materials. "Physics for scientists and engineers," 4th edition, Vol.1, Raymond A. Serway, Saunders College Publishing, 1996. Ex. 1) When wood burns, mass seems to disappear because some of the products of reaction are gases; if the mass of the original wood is added to the mass of the oxygen that combined with it and if the mass of the resulting ash is added to the mass o the gaseous products, the two sums will turn out exactly equal. 2) Iron increases in weight on rusting because it combines with gases from the air, and the increase in weight is exactly equal to the weight of gas consumed. Out of thousands of reactions that have been tested with accurate chemical balances, no deviation from the law has ever been found. Law of Conversation of Energy: The total energy of a closed system is constant. Matter is neither created nor destroyed – total mass of reactants equals total mass of products You can calculate the change of temp by simply understanding that energy and the mass is conserved - it means that we added the two heat quantities together we can calculate the change of temperature by using the law or measure change of temp and show the conservation of energy E1 + E2 = E3 -> E(universe) = E(System) + E(Surroundings) M1 + M2 = M3 Is T1 + T2 = unknown (No, no law of conservation of temperature, so we have to use the concept of conservation of energy) Total amount of thermal energy in beaker of water in absolute terms as opposed to differential terms (reference point is 0 degrees Kelvin) Knowns: M1, M2, T1, T2 (Kelvin) When add the two together, want to know what T3 and M3 are going to be. -

Uniqueness of Solution for the 2D Primitive Equations with Friction Condition on the Bottom∗ 1 Introduction and Motivation
Monograf´ıas del Semin. Matem. Garc´ıa de Galdeano. 27: 135{143, (2003). Uniqueness of solution for the 2D Primitive Equations with friction condition on the bottom∗ D. Breschy, F. Guill´en-Gonz´alezz, N. Masmoudix, M. A. Rodr´ıguez-Bellido{ Abstract Uniqueness of solution for the Primitive Equations with Dirichlet conditions on the bottom is an open problem even in 2D domains. In this work we prove a result of additional regularity for a weak solution v for the Primitive Equations when we replace Dirichlet boundary conditions by friction conditions. This allows to obtain uniqueness of weak solution global in time, for such a system [3]. Indeed, we show weak regularity for the vertical derivative of the solution, @zv for all time. This is because this derivative verifies a linear pde of convection-diffusion type with convection velocity v, and the pressure belongs to a L2-space in time with values in a weighted space. Keywords: Boundary conditions of type Navier, 2D Primitive Equations, uniqueness AMS Classification: 35Q30, 35B40, 76D05 1 Introduction and motivation. Primitive Equations are one of the models used to forecast the fluid velocity and pressure in the ocean. Such equations are obtained from the dimensionless Navier-Stokes equations, letting the aspect ratio (quotient between vertical dimension and horizontal dimensions) go to zero. The first results about existence of solution (weak, in the sense of the Navier- Stokes equations) are proved for boundary conditions of Dirichlet type on the bottom of the domain and with wind traction on the surface, in the works by Lions-Temam-Wang, ∗The second and fourth authors have been financed by the C.I.C.Y.T project MAR98-0486. -

Shallow-Water Equations and Related Topics
CHAPTER 1 Shallow-Water Equations and Related Topics Didier Bresch UMR 5127 CNRS, LAMA, Universite´ de Savoie, 73376 Le Bourget-du-Lac, France Contents 1. Preface .................................................... 3 2. Introduction ................................................. 4 3. A friction shallow-water system ...................................... 5 3.1. Conservation of potential vorticity .................................. 5 3.2. The inviscid shallow-water equations ................................. 7 3.3. LERAY solutions ........................................... 11 3.3.1. A new mathematical entropy: The BD entropy ........................ 12 3.3.2. Weak solutions with drag terms ................................ 16 3.3.3. Forgetting drag terms – Stability ............................... 19 3.3.4. Bounded domains ....................................... 21 3.4. Strong solutions ............................................ 23 3.5. Other viscous terms in the literature ................................. 23 3.6. Low Froude number limits ...................................... 25 3.6.1. The quasi-geostrophic model ................................. 25 3.6.2. The lake equations ...................................... 34 3.7. An interesting open problem: Open sea boundary conditions .................... 41 3.8. Multi-level and multi-layers models ................................. 43 3.9. Friction shallow-water equations derivation ............................. 44 3.9.1. Formal derivation ....................................... 44 3.10. -

Heat and Energy Conservation
1 Lecture notes in Fluid Dynamics (1.63J/2.01J) by Chiang C. Mei, MIT, Spring, 2007 CHAPTER 4. THERMAL EFFECTS IN FLUIDS 4-1-2energy.tex 4.1 Heat and energy conservation Recall the basic equations for a compressible fluid. Mass conservation requires that : ρt + ∇ · ρ~q = 0 (4.1.1) Momentum conservation requires that : = ρ (~qt + ~q∇ · ~q)= −∇p + ∇· τ +ρf~ (4.1.2) = where the viscous stress tensor τ has the components = ∂qi ∂qi ∂qk τ = τij = µ + + λ δij ij ∂xj ∂xi ! ∂xk There are 5 unknowns ρ, p, qi but only 4 equations. One more equation is needed. 4.1.1 Conservation of total energy Consider both mechanical ad thermal energy. Let e be the internal (thermal) energy per unit mass due to microscopic motion, and q2/2 be the kinetic energy per unit mass due to macroscopic motion. Conservation of energy requires D q2 ρ e + dV rate of incr. of energy in V (t) Dt ZZZV 2 ! = − Q~ · ~ndS rate of heat flux into V ZZS + ρf~ · ~qdV rate of work by body force ZZZV + Σ~ · ~qdS rate of work by surface force ZZX Use the kinematic transport theorm, the left hand side becomes D q2 ρ e + dV ZZZV Dt 2 ! 2 Using Gauss theorem the heat flux term becomes ∂Qi − QinidS = − dV ZZS ZZZV ∂xi The work done by surface stress becomes Σjqj dS = (σjini)qj dS ZZS ZZS ∂(σijqj) = (σijqj)ni dS = dV ZZS ZZZV ∂xi Now all terms are expressed as volume integrals over an arbitrary material volume, the following must be true at every point in space, 2 D q ∂Qi ∂(σijqi) ρ e + = − + ρfiqi + (4.1.3) Dt 2 ! ∂xi ∂xj As an alternative form, we differentiate the kinetic energy and get De -

Lecture 5: Magnetic Mirroring
!"#$%&'()%"*#%*+,-./-*+01.2(.*3+456789* !"#$%&"'()'*+,-".#'*/&&0&/-,' Dr. Peter T. Gallagher Astrophysics Research Group Trinity College Dublin :&2-;-)(*!"<-$2-"(=*%>*/-?"=)(*/%/="#* o Gyrating particle constitutes an electric current loop with a dipole moment: 1/2mv2 µ = " B o The dipole moment is conserved, i.e., is invariant. Called the first adiabatic invariant. ! o µ = constant even if B varies spatially or temporally. If B varies, then vperp varies to keep µ = constant => v|| also changes. o Gives rise to magnetic mirroring. Seen in planetary magnetospheres, magnetic bottles, coronal loops, etc. Bz o Right is geometry of mirror Br from Chen, Page 30. @-?"=)(*/2$$%$2"?* o Consider B-field pointed primarily in z-direction and whose magnitude varies in z- direction. If field is axisymmetric, B! = 0 and d/d! = 0. o This has cylindrical symmetry, so write B = Brrˆ + Bzzˆ o How does this configuration give rise to a force that can trap a charged particle? ! o Can obtain Br from " #B = 0 . In cylindrical polar coordinates: 1 " "Bz (rBr )+ = 0 r "r "z ! " "Bz => (rBr ) = #r "r "z o If " B z / " z is given at r = 0 and does not vary much with r, then r $Bz 1 2&$ Bz ) ! rBr = " #0 r dr % " r ( + $z 2 ' $z *r =0 ! 1 &$ Bz ) Br = " r( + (5.1) 2 ' $z *r =0 ! @-?"=)(*/2$$%$2"?* o Now have Br in terms of BZ, which we can use to find Lorentz force on particle. o The components of Lorentz force are: Fr = q(v" Bz # vzB" ) (1) F" = q(#vrBz + vzBr ) (2) (3) Fz = q(vrB" # v" Br ) (4) o As B! = 0, two terms vanish and terms (1) and (2) give rise to Larmor gyration. -

Global Well-Posedness of the Three-Dimensional Viscous Primitive Equations of Large Scale Ocean and Atmosphere Dynamics
Annals of Mathematics, 166 (2007), 245–267 Global well-posedness of the three-dimensional viscous primitive equations of large scale ocean and atmosphere dynamics By Chongsheng Cao and Edriss S. Titi Abstract In this paper we prove the global existence and uniqueness (regularity) of strong solutions to the three-dimensional viscous primitive equations, which model large scale ocean and atmosphere dynamics. 1. Introduction Large scale dynamics of oceans and atmosphere is governed by the primi- tive equations which are derived from the Navier-Stokes equations, with rota- tion, coupled to thermodynamics and salinity diffusion-transport equations, which account for the buoyancy forces and stratification effects under the Boussinesq approximation. Moreover, and due to the shallowness of the oceans and the atmosphere, i.e., the depth of the fluid layer is very small in compar- ison to the radius of the earth, the vertical large scale motion in the oceans and the atmosphere is much smaller than the horizontal one, which in turn leads to modeling the vertical motion by the hydrostatic balance. As a result one obtains the system (1)–(4), which is known as the primitive equations for ocean and atmosphere dynamics (see, e.g., [20], [21], [22], [23], [24], [33] and references therein). We observe that in the case of ocean dynamics one has to add the diffusion-transport equation of the salinity to the system (1)–(4). We omitted it here in order to simplify our mathematical presentation. However, we emphasize that our results are equally valid when the salinity effects are taking into account. Note that the horizontal motion can be further approximated by the geostrophic balance when the Rossby number (the ratio of the horizontal ac- celeration to the Coriolis force) is very small. -

Chapter 15 - Fluid Mechanics Thursday, March 24Th
Chapter 15 - Fluid Mechanics Thursday, March 24th •Fluids – Static properties • Density and pressure • Hydrostatic equilibrium • Archimedes principle and buoyancy •Fluid Motion • The continuity equation • Bernoulli’s effect •Demonstration, iClicker and example problems Reading: pages 243 to 255 in text book (Chapter 15) Definitions: Density Pressure, ρ , is defined as force per unit area: Mass M ρ = = [Units – kg.m-3] Volume V Definition of mass – 1 kg is the mass of 1 liter (10-3 m3) of pure water. Therefore, density of water given by: Mass 1 kg 3 −3 ρH O = = 3 3 = 10 kg ⋅m 2 Volume 10− m Definitions: Pressure (p ) Pressure, p, is defined as force per unit area: Force F p = = [Units – N.m-2, or Pascal (Pa)] Area A Atmospheric pressure (1 atm.) is equal to 101325 N.m-2. 1 pound per square inch (1 psi) is equal to: 1 psi = 6944 Pa = 0.068 atm 1atm = 14.7 psi Definitions: Pressure (p ) Pressure, p, is defined as force per unit area: Force F p = = [Units – N.m-2, or Pascal (Pa)] Area A Pressure in Fluids Pressure, " p, is defined as force per unit area: # Force F p = = [Units – N.m-2, or Pascal (Pa)] " A8" rea A + $ In the presence of gravity, pressure in a static+ 8" fluid increases with depth. " – This allows an upward pressure force " to balance the downward gravitational force. + " $ – This condition is hydrostatic equilibrium. – Incompressible fluids like liquids have constant density; for them, pressure as a function of depth h is p p gh = 0+ρ p0 = pressure at surface " + Pressure in Fluids Pressure, p, is defined as force per unit area: Force F p = = [Units – N.m-2, or Pascal (Pa)] Area A In the presence of gravity, pressure in a static fluid increases with depth. -

Fluid Dynamics and Mantle Convection
Subduction II Fundamentals of Mantle Dynamics Thorsten W Becker University of Southern California Short course at Universita di Roma TRE April 18 – 20, 2011 Rheology Elasticity vs. viscous deformation η = O (1021) Pa s = viscosity µ= O (1011) Pa = shear modulus = rigidity τ = η / µ = O(1010) sec = O(103) years = Maxwell time Elastic deformation In general: σ ε ij = Cijkl kl (in 3-D 81 degrees of freedom, in general 21 independent) For isotropic body this reduces to Hooke’s law: σ λε δ µε ij = kk ij + 2 ij λ µ ε ε ε ε with and Lame’s parameters, kk = 11 + 22 + 33 Taking shear components ( i ≠ j ) gives definition of rigidity: σ µε 12 = 2 12 Adding the normal components ( i=j ) for all i=1,2,3 gives: σ λ µ ε κε kk = (3 + 2 ) kk = 3 kk with κ = λ + 2µ/3 = bulk modulus Linear viscous deformation (1) Total stress field = static + dynamic part: σ = − δ +τ ij p ij ij Analogous to elasticity … General case: τ = ε ij C'ijkl kl Isotropic case: τ = λ ε δ + ηε ij ' kk ij 2 ij Linear viscous deformation (2) Split in isotropic and deviatoric part (latter causes deformation): 1 σ ' = σ − σ δ = σ + pδ ij ij 3 kk ij ij ij 1 ε' = ε − ε δ ij ij 3 kk ij which gives the following stress: σ = − δ + ςε δ + ηε 'ij ( p p) ij kk ij 2 'ij ε = With compressibility term assumed 0 (Stokes condition kk 0 ) 2 ( ς = λ ' + η = bulk viscosity) 3 τ Now τ = 2 η ε or η = ij ij ij ε 2 ij η = In general f (T,d, p, H2O) Non-linear (or non-Newtonian) deformation General stress-strain relation: ε = Aτ n n = 1 : Newtonian n > 1 : non-Newtonian n → ∞ : pseudo-brittle 1 −1 1−n 1 −1/ n (1−n) / n Effective viscosity η = A τ = A ε eff 2 2 Application: different viscosities under oceans with different absolute plate motion, anisotropic viscosities by means of superposition (Schmeling, 1987) Microphysical theory and observations Maximum strength of materials (1) ‘Strength’ is maximum stress that material can resist In principle, viscous fluid has zero strength. -

Experiment 7: Conservation of Energy
Experiment 7: Conservation of Energy One of the most important and useful concepts in mechanics is that of \Conservation of Energy". In this experiment, you will make measurements to demonstrate the conservation of mechanical energy and its transformation between kinetic energy and potential energy. The Total Mechanical Energy, E, of a system is defined as the sum of the Kinetic Energy, K, and the Potential Energy, U: E = K + U: (1) If the system is isolated, with only conservative forces acting on its parts, the total mechanical energy of the system is a constant. The mechanical energy can, however, be transformed between its kinetic and potential forms. cannot be destroyed. Rather, the energy is transmitted from one form to another. Any change in the kinetic energy will cause a corresponding change in the potential energy, and vice versa. The conservation of energy then dictates that, ∆K + ∆U = 0 (2) where ∆K is the change in the kinetic energy, and ∆U is the change in potential energy. Potential energy is a form of stored energy and is a consequence of the work done by a force. Examples of forces which have an associated potential energy are the gravitational and the electromagnetic fields and, in mechanics, a spring. In a sense potential energy is a storage system for energy. For a body moving under the influence of a force F , the change in potential energy is given by Z f −! −! ∆U = − F · ds (3) i where i and f represent the initial and final positions of the body, respectively. Hence, from Equation 2 we have what is commonly referred to as the work-kinetic energy theorem: Z f −! −! ∆K = F · ds (4) i Consider a body of mass m, being accelerated by a compressed spring.