Morphophysiological Constraints on Foregut, and Adaptations of Hindgut Fermenters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Equine Laminitis Managing Pasture to Reduce the Risk
Equine Laminitis Managing pasture to reduce the risk RIRDCnew ideas for rural Australia © 2010 Rural Industries Research and Development Corporation. All rights reserved. ISBN 978 1 74254 036 8 ISSN 1440-6845 Equine Laminitis - Managing pasture to reduce the risk Publication No. 10/063 Project No.PRJ-000526 The information contained in this publication is intended for general use to assist public knowledge and discussion and to help improve the development of sustainable regions. You must not rely on any information contained in this publication without taking specialist advice relevant to your particular circumstances. While reasonable care has been taken in preparing this publication to ensure that information is true and correct, the Commonwealth of Australia gives no assurance as to the accuracy of any information in this publication. The Commonwealth of Australia, the Rural Industries Research and Development Corporation (RIRDC), the authors or contributors expressly disclaim, to the maximum extent permitted by law, all responsibility and liability to any person, arising directly or indirectly from any act or omission, or for any consequences of any such act or omission, made in reliance on the contents of this publication, whether or not caused by any negligence on the part of the Commonwealth of Australia, RIRDC, the authors or contributors. The Commonwealth of Australia does not necessarily endorse the views in this publication. This publication is copyright. Apart from any use as permitted under the Copyright Act 1968, all other rights are reserved. However, wide dissemination is encouraged. Requests and inquiries concerning reproduction and rights should be addressed to the RIRDC Publications Manager on phone 02 6271 4165. -

Guidebook Contains Preliminary Findings of a Number of Concurrent Projects Being Worked on by the Trip Leaders
TH FRIENDS OF THE PLEISTOCENE, ROCKY MOUNTAIN-CELL, 45 FIELD CONFERENCE PLIO-PLEISTOCENE STRATIGRAPHY AND GEOMORPHOLOGY OF THE CENTRAL PART OF THE ALBUQUERQUE BASIN OCTOBER 12-14, 2001 SEAN D. CONNELL New Mexico Bureau of Geology and Mineral Resources-Albuquerque Office, New Mexico Institute of Mining and Technology, 2808 Central Ave. SE, Albuquerque, New Mexico 87106 DAVID W. LOVE New Mexico Bureau of Geology and Mineral Resources, New Mexico Institute of Mining and Technology, 801 Leroy Place, Socorro, NM 87801 JOHN D. SORRELL Tribal Hydrologist, Pueblo of Isleta, P.O. Box 1270, Isleta, NM 87022 J. BRUCE J. HARRISON Dept. of Earth and Environmental Sciences, New Mexico Institute of Mining and Technology 801 Leroy Place, Socorro, NM 87801 Open-File Report 454C and D Initial Release: October 11, 2001 New Mexico Bureau of Geology and Mineral Resources New Mexico Institute of Mining and Technology 801 Leroy Place, Socorro, NM 87801 NMBGMR OFR454 C & D INTRODUCTION This field-guide accompanies the 45th annual Rocky Mountain Cell of the Friends of the Pleistocene (FOP), held at Isleta Lakes, New Mexico. The Friends of the Pleistocene is an informal gathering of Quaternary geologists, geomorphologists, and pedologists who meet annually in the field. The field guide has been separated into two parts. Part C (open-file report 454C) contains the three-days of road logs and stop descriptions. Part D (open-file report 454D) contains a collection of mini-papers relevant to field-trip stops. This field guide is a companion to open-file report 454A and 454B, which accompanied a field trip for the annual meeting of the Rocky Mountain/South Central Section of the Geological Society of America, held in Albuquerque in late April. -

Middle Miocene Paleoenvironmental Reconstruction of the Central Great Plains from Stable Carbon Isotopes in Large Mammals Willow H
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Dissertations & Theses in Earth and Atmospheric Earth and Atmospheric Sciences, Department of Sciences 7-2017 Middle Miocene Paleoenvironmental Reconstruction of the Central Great Plains from Stable Carbon Isotopes in Large Mammals Willow H. Nguy University of Nebraska-Lincoln, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/geoscidiss Part of the Geology Commons, Paleobiology Commons, and the Paleontology Commons Nguy, Willow H., "Middle Miocene Paleoenvironmental Reconstruction of the Central Great Plains from Stable Carbon Isotopes in Large Mammals" (2017). Dissertations & Theses in Earth and Atmospheric Sciences. 91. http://digitalcommons.unl.edu/geoscidiss/91 This Article is brought to you for free and open access by the Earth and Atmospheric Sciences, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Dissertations & Theses in Earth and Atmospheric Sciences by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. MIDDLE MIOCENE PALEOENVIRONMENTAL RECONSTRUCTION OF THE CENTRAL GREAT PLAINS FROM STABLE CARBON ISOTOPES IN LARGE MAMMALS by Willow H. Nguy A THESIS Presented to the Faculty of The Graduate College at the University of Nebraska In Partial Fulfillment of Requirements For the Degree of Master of Science Major: Earth and Atmospheric Sciences Under the Supervision of Professor Ross Secord Lincoln, Nebraska July, 2017 MIDDLE MIOCENE PALEOENVIRONMENTAL RECONSTRUCTION OF THE CENTRAL GREAT PLAINS FROM STABLE CARBON ISOTOPES IN LARGE MAMMALS Willow H. Nguy, M.S. University of Nebraska, 2017 Advisor: Ross Secord Middle Miocene (18-12 Mya) mammalian faunas of the North American Great Plains contained a much higher diversity of apparent browsers than any modern biome. -

Prospects for Rewilding with Camelids
Journal of Arid Environments 130 (2016) 54e61 Contents lists available at ScienceDirect Journal of Arid Environments journal homepage: www.elsevier.com/locate/jaridenv Prospects for rewilding with camelids Meredith Root-Bernstein a, b, *, Jens-Christian Svenning a a Section for Ecoinformatics & Biodiversity, Department of Bioscience, Aarhus University, Aarhus, Denmark b Institute for Ecology and Biodiversity, Santiago, Chile article info abstract Article history: The wild camelids wild Bactrian camel (Camelus ferus), guanaco (Lama guanicoe), and vicuna~ (Vicugna Received 12 August 2015 vicugna) as well as their domestic relatives llama (Lama glama), alpaca (Vicugna pacos), dromedary Received in revised form (Camelus dromedarius) and domestic Bactrian camel (Camelus bactrianus) may be good candidates for 20 November 2015 rewilding, either as proxy species for extinct camelids or other herbivores, or as reintroductions to their Accepted 23 March 2016 former ranges. Camels were among the first species recommended for Pleistocene rewilding. Camelids have been abundant and widely distributed since the mid-Cenozoic and were among the first species recommended for Pleistocene rewilding. They show a range of adaptations to dry and marginal habitats, keywords: Camelids and have been found in deserts, grasslands and savannas throughout paleohistory. Camelids have also Camel developed close relationships with pastoralist and farming cultures wherever they occur. We review the Guanaco evolutionary and paleoecological history of extinct and extant camelids, and then discuss their potential Llama ecological roles within rewilding projects for deserts, grasslands and savannas. The functional ecosystem Rewilding ecology of camelids has not been well researched, and we highlight functions that camelids are likely to Vicuna~ have, but which require further study. -

Why Species of Grazing Animal Is Important
Why Species of Grazing Animal is Important Based on: Grazers and Browsers: How Digestive Morphology Affects Diet Selection. 1999. By Lisa Shipley. Available at: www.cnr.uidaho.edu/range/pubs/Behavior/Shipley.pdf The species of grazing animal determines: What the animal is likely to eat . Grasses/Roughage (Grazers) . Browses (Browsers) . Mix of Grass, Forbs, and Browse (Intermediate Feeders) The animal’s ability to use terrain Transmission of disease between species Amount of water needed and frequency Start by knowing if the critter in question eats meat of plants: Herbivore Species - Page 1 Mammalian Digestive System– Makes the difference o Digestive system anatomy: Rumen - site of fermentation (absorb Volatile Fatty Acids and Ammonia) True Stomach (abomasum in ruminants) - secrets enzyme for digestion Small Intestines - absorption of nutrients Cecum - site of fermentation (absorb VFA's and Ammonia) Can The Animal Digest Cellulose? – An important distinction Carnivore ↔ Omnivores ↔ Herbivores NO ↔ SOMEWHAT ↔ NO o Limited Cellulose Digestion- Monogastrics: Swine, birds, reptiles No rumen or large cecum-colon for cellulose fermentation Get energy from simple CHO's (sugars) and starch. o Cellulose Digestion: Enlarged Fermentation Organ . Which houses microbes (bacteria and protozoa) These microbes break to bonds of cellulose and release VFA's (volatile fatty acids) as a byproduct. The VFA's are absorbed through the rumen or cecum wall where they are transported to the liver and converted to "things" that can be used for energy by the animal (glucose, acetyl coA, oxyacetyl acid and fat). Foregut Digestion: including Ruminants and Camilids . For Example: Cows, sheep, deer, bison, elk, pronghorn . Have a rumen for fermentation Herbivore Species - Page 2 . -

Morton County, Kansas Michael Anthony Calvello Fort Hays State University
Fort Hays State University FHSU Scholars Repository Master's Theses Graduate School Summer 2011 Mammalian Fauna From The ulF lerton Gravel Pit (Ogallala Group, Late Miocene), Morton County, Kansas Michael Anthony Calvello Fort Hays State University Follow this and additional works at: https://scholars.fhsu.edu/theses Part of the Geology Commons Recommended Citation Calvello, Michael Anthony, "Mammalian Fauna From The ulF lerton Gravel Pit (Ogallala Group, Late Miocene), Morton County, Kansas" (2011). Master's Theses. 135. https://scholars.fhsu.edu/theses/135 This Thesis is brought to you for free and open access by the Graduate School at FHSU Scholars Repository. It has been accepted for inclusion in Master's Theses by an authorized administrator of FHSU Scholars Repository. - MAMMALIAN FAUNA FROM THE FULLERTON GRAVEL PIT (OGALLALA GROUP, LATE MIOCENE), MORTON COUNTY, KANSAS being A Thesis Presented to the Graduate Faculty of the Fort Hays State University in Partial Fulfillment of the Requirements for the Degree of Master of Science by Michael Anthony Calvello B.A., State University of New York at Geneseo Date Z !J ~4r u I( Approved----c-~------------- Chair, Graduate Council Graduate Committee Approval The Graduate Committee of Michael A. Calvello hereby approves his thesis as meeting partial fulfillment of the requirement for the Degree of Master of Science. Approved: )<--+-- ----+-G~IJCommittee Meilnber Approved: ~J, iii,,~ Committee Member 11 ABSTRACT The Fullerton Gravel Pit, Morton County, Kansas is one of many sites in western Kansas at which the Ogallala Group crops out. The Ogallala Group was deposited primarily by streams flowing from the Rocky Mountains. Evidence of water transport is observable at the Fullerton Gravel Pit through the presence of allochthonous clasts, cross-bedding, pebble alignment, and fossil breakage and subsequent rounding. -

Southern Exposures
Searching for the Pliocene: Southern Exposures Robert E. Reynolds, editor California State University Desert Studies Center The 2012 Desert Research Symposium April 2012 Table of contents Searching for the Pliocene: Field trip guide to the southern exposures Field trip day 1 ���������������������������������������������������������������������������������������������������������������������������������������������� 5 Robert E. Reynolds, editor Field trip day 2 �������������������������������������������������������������������������������������������������������������������������������������������� 19 George T. Jefferson, David Lynch, L. K. Murray, and R. E. Reynolds Basin thickness variations at the junction of the Eastern California Shear Zone and the San Bernardino Mountains, California: how thick could the Pliocene section be? ��������������������������������������������������������������� 31 Victoria Langenheim, Tammy L. Surko, Phillip A. Armstrong, Jonathan C. Matti The morphology and anatomy of a Miocene long-runout landslide, Old Dad Mountain, California: implications for rock avalanche mechanics �������������������������������������������������������������������������������������������������� 38 Kim M. Bishop The discovery of the California Blue Mine ��������������������������������������������������������������������������������������������������� 44 Rick Kennedy Geomorphic evolution of the Morongo Valley, California ���������������������������������������������������������������������������� 45 Frank Jordan, Jr. New records -

Impact of Starch Source on Equine Hindgut Microbial Ecology
University of Kentucky UKnowledge Theses and Dissertations--Animal and Food Sciences Animal and Food Sciences 2015 IMPACT OF STARCH SOURCE ON EQUINE HINDGUT MICROBIAL ECOLOGY Brittany Elizabeth Davis Harlow University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Harlow, Brittany Elizabeth Davis, "IMPACT OF STARCH SOURCE ON EQUINE HINDGUT MICROBIAL ECOLOGY" (2015). Theses and Dissertations--Animal and Food Sciences. 55. https://uknowledge.uky.edu/animalsci_etds/55 This Doctoral Dissertation is brought to you for free and open access by the Animal and Food Sciences at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Animal and Food Sciences by an authorized administrator of UKnowledge. For more information, please contact [email protected]. STUDENT AGREEMENT: I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained needed written permission statement(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine) which will be submitted to UKnowledge as Additional File. I hereby grant to The University of Kentucky and its agents the irrevocable, non-exclusive, and royalty-free license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known. -

Giant Camels from the Cenozoic of North America SERIES PUBLICATIONS of the SMITHSONIAN INSTITUTION
Giant Camels from the Cenozoic of North America SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the H^arine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Folklife Studies Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that refXJrt the research and collections of its various museums and bureaux or of professional colleagues in the world of science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the worid. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. Press requirements for manuscript and art preparation are outlined on the inside back cover. -

Multivariate Discriminant Function Analysis of Camelid Astragali
Palaeontologia Electronica palaeo-electronica.org A method for improved identification of postcrania from mammalian fossil assemblages: multivariate discriminant function analysis of camelid astragali Edward Byrd Davis and Brianna K. McHorse ABSTRACT Character-rich craniodental specimens are often the best material for identifying mammalian fossils to the genus or species level, but what can be done with the many assemblages that consist primarily of dissociated postcrania? In localities lacking typi- cally diagnostic remains, accurate identification of postcranial material can improve measures of mammalian diversity for wider-scale studies. Astragali, in particular, are often well-preserved and have been shown to have diagnostic utility in artiodactyls. The Thousand Creek fauna of Nevada (~8 Ma) represents one such assemblage rich in postcranial material but with unknown diversity of many taxa, including camelids. We use discriminant function analysis (DFA) of eight linear measurements on the astragali of contemporaneous camelids with known taxonomic affinity to produce a training set that can then be used to assign taxa to the Thousand Creek camelid material. The dis- criminant function identifies, at minimum, four classes of camels: “Hemiauchenia”, Alforjas, Procamelus, and ?Megatylopus. Adding more specimens to the training set may improve certainty and accuracy for future work, including identification of camelids in other faunas of similar age. For best statistical practice and ease of future use, we recommend using DFA rather than qualitative analyses of biplots to separate and diag- nose taxa. Edward Byrd Davis. University of Oregon Museum of Natural and Cultural History and Department of Geological Sciences, 1680 East 15th Avenue, Eugene, Oregon 97403. [email protected] Brianna K. -

The Effect of a Hindgut Modifier Pelleted Product Containing Saccharomyces Cerevisiae on Cecal Ph in the Equine Hindgut Megan Mary Hall Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1-1-2005 The effect of a hindgut modifier pelleted product containing Saccharomyces cerevisiae on cecal pH in the equine hindgut Megan Mary Hall Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Recommended Citation Hall, Megan Mary, "The effect of a hindgut modifier pelleted product containing Saccharomyces cerevisiae on cecal pH in the equine hindgut" (2005). Retrospective Theses and Dissertations. 18803. https://lib.dr.iastate.edu/rtd/18803 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. The effect of a hindgut modifier pelleted product containing Saccharomyces cerevisiae on cecal pH in the equine hindgut by Megan Mary Hall A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Animal Physiology (Reproductive Physiology) Program of Study Committee: Peggy A. Miller-Auwerda, Major Professor Howard D. Tyler Richard B. Evans Iowa State University Ames, Iowa 2005 11 Graduate College Iowa State University This is to certify that the master's thesis of Megan Mary Hall has met the thesis requirements of Iowa State University Signatures have been redacted for privacy 111 DEDICATION This thesis is dedicated to my parents, Larry Edwin Hall and Claire Ann Lindgren Hall for their continual support and for pushing me to strive to be the best in everything I do. -
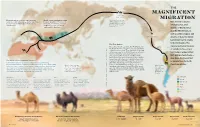
Magnificent Migration
THE MAGNIFICENT Land bridge 8 MY–14,500 Y MIGRATION Domestication of camels began between World camel population today About 6 million years ago, 3,000 and 4,000 years ago—slightly later than is about 30 million: 27 million of camelids began to move Best known today for horses—in both the Arabian Peninsula and these are dromedaries; 3 million westward across the land Camelini western Asia. are Bactrians; and only about that connected Asia and inhabiting hot, arid North America. 1,000 are Wild Bactrians. regions of North Africa and the Middle East, as well as colder steppes and Camelid ancestors deserts of Asia, the family Camelidae had its origins in North America. The The First Camels signature physical features The earliest-known camelids, the Protylopus and Lamini E the Poebrotherium, ranged in sizes comparable to of camels today—one or modern hares to goats. They appeared roughly 40 DMOR I SK million years ago in the North American savannah. two humps, wide padded U L Over the 20 million years that followed, more U L ; feet, well-protected eyes— O than a dozen other ancestral members of the T T O family Camelidae grew, developing larger bodies, . E may have developed K longer legs and long necks to better browse high C I The World's Most Adaptable Traveler? R ; vegetation. Some, like Megacamelus, grew even I as adaptations to North Camels have adapted to some of the Earth’s most demanding taller than the woolly mammoths in their time. LEWSK (Later, in the Middle East, the Syrian camel may American winters.