A Study of Absorption Spectrum of the Local Product of Amber (Payin) in Kachin State
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Title == Red/Amber Report
DOT HS 811 115 April 2009 The Effectiveness of Amber Rear Turn Signals for Reducing Rear Impacts This report is free of charge from the NHTSA Web site at www.nhtsa.dot.gov This publication is distributed by the U.S. Department of Transportation, National Highway Traffic Safety Administration, in the interest of information exchange. The opinions, findings, and conclusions expressed in this publication are those of the author and not necessarily those of the Department of Transportation or the National Highway Traffic Safety Administration. The United States Government assumes no liability for its content or use thereof. If trade or manufacturer’s names or products are mentioned, it is because they are considered essential to the object of the publication and should not be construed as an endorsement. The United States Government does not endorse products or manufacturers. Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient’s Catalog No. DOT HS 811 115 4. Title and Subtitle 5. Report Date The Effectiveness of Amber Rear Turn Signals for Reducing Rear April 2009 Impacts 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Kirk Allen, Ph.D. 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Evaluation Division; National Center for Statistics and Analysis National Highway Traffic Safety Administration 11. Contract or Grant No. Washington, DC 20590 12. Sponsoring Agency Name and Address 13. Type of Report and Period Covered National Highway Traffic Safety Administration NHTSA Technical Report 1200 New Jersey Avenue SE. 14. Sponsoring Agency Code Washington, DC 20590 15. -

Mid Atlantic Carnival Glass Jamboree Thursday October 17 and Saturday October 19, 2019
Mid Atlantic Carnival Glass Jamboree Thursday October 17 and Saturday October 19, 2019 Thursday Day One; Lots 1-175 ____1. Fenton Contemporary Red Diamond Rib Basket. ____29. NORTHWOOD BLUE POPPY SHOW PLATE. ____2. 1987 ACGA Green Morning Glory Pitcher Souvenir. (NICE EXAMPLE.) ____3. Pair of 1998 & 2000 HOACGA Room Display Awards. ____30. NORTHWOOD GREEN POPPY SHOW PLATE. (Clear Opal and Blue Hobnail Spittoons.) (VERY NICE EXAMPLE.) ____4. 1991 & 1996 ACGA Red Inverted Strawberry ____31. Northwood Purple Strawberry Plate. (Has Basketweave Souvenirs. (Handled Basket and Creamer.) Exterior and Good Iridescence.) ____5. 2001 ACGA Vaseline Opal Seacoast Spittoon Souvenir. ____32. Northwood Blue Three Fruits Stippled PCE Bowl. ____6. (3) ACGA Green Opal Seacoast Spittoon Souvenirs. (Ribbed Exterior. Nice Iridescence.) (Columbus Ohio.) ____33. Northwood Green Greek Key PCE Bowl. ____7. Fenton Contemporary Bulbous Vase. (Tri-Cornered (Has Basketweave Exterior.) Crimped Edge.) ____34. Northwood Purple Good Luck Ruffled Bowl. ____8. 1998 & 1999 HOACGA Souvenirs. (White Loving Cup (Ribbed Exterior.) and Vaseline Opal Fine Cut & Roses Rosebowl.) ____35. NORTHWOOD GREEN PEACOCK AT THE URN ____9. Imperial Electric Purple Water Carafe & Wine Decanter. MASTER ICS BOWL. (VERY NICE EXAMPLE; RARE (Decanter Has No Stopper.) COLOR.) ____10. IMPERIAL ELECTRIC PURPLE BROKEN ARCHES ____36. NORTHWOOD BLUE PEACOCK AT THE URN PUNCH BOWL. (GORGEOUS IRIDESCENCE AND MASTER ICS BOWL. (NICE ELECTRIC PRETTY DARN EVEN.) HIGHLIGHTS.) ____11. (3) Imperial Purple Broken Arches Punch Cups. ____37. Northwood Amethyst Peacock at the Urn Master ICS ____12. Imperial Electric Purple Diamond Lace 5 PC. Water Set. Bowl. (One Tumbler Has Edge Nick as Seen in Photos.) ____38. Northwood Dark Marigold Peacock at the Urn Master ____13. -

Elektrabar Mini (8) 12W RGBWAI LED Linear Strip
ELE815 ElektraBar Mini (8) 12W RGBWAI LED Linear Strip GENERAL INFORMATION A powerful, full‐featured linear strip fixture, the elektraLite ElektraBar Mini is ready to bring rich vibrant color at a professional level. At the heart of the elektraLite ElektraBar Mini are eight powerful 12‐watt 6‐in‐1 LED’s, consisting of a red, green, blue, white, amber, and indigo component. The elektraBar Mini features individual control of each pixel, allowing for 16 million colors on eight different LED pixels. With precision spacing, the fixture is also ideal for low‐resolution pixel mapping and video wall applications. The elektraLite elektraBar Mini is designed to deliver rich, vibrant colors you expect from an LED fixture, while still d providing true, brilliant, tunable white. By mixing color internally, the ElektraBar Mini’s sophisticated diffraction lensing system provide true, blended, single‐color output. Because of this, the color range for pastel and deep blues and purples is intensified with the indigo component. FEATURES ORDERING INFORMATION ELE815 ElektraBar Mini Linear Strip 25° (black) ‐ utilizes eight individually controllable 12‐watt 6‐IN‐1 LED’s ELE815‐10 10° Lens Kit (set of 8) ‐ rich, vivid red, blue, green and white, with added amber for ELE815‐40 40° Lens Kit (set of 8) true white color toning, and indigo for pastel blending and ELE815‐GS 4” adjustable Glare Shield / Beam Flange well‐rounded color saturation ELE724‐AP* 12” Edison to IP68 Power Adapter ‐ individual control of each pixel, allowing 16 million colors ELE724‐AD* 12” -
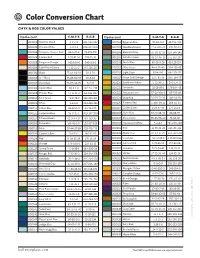
Color Conversion Chart
Color Conversion Chart CMYK & RGB COLOR VALUES Opalescent C-M-Y-K R-G-B Opalescent C-M-Y-K R-G-B 000009 Reactive Cloud 4-2-1-0 241-243-247 000164 Egyptian Blue 81-48-0-0 49-116-184 000013 Opaque White 4-2-2-1 246-247-249 000203 Woodland Brown 22-63-87-49 120-70-29 000016 Turquoise Opaque Rod 65-4-27-6 75-174-179 000206 Elephant Gray 35-30-32-18 150-145-142 000024 Tomato Red 1-99-81-16 198-15-36 000207 Celadon Green 43-14-46-13 141-167-137 000025 Tangerine Orange 1-63-100-0 240-119-2 000208 Dusty Blue 60-25-9-28 83-123-154 000034 Light Peach Cream 5-12-15-0 243-226-213 000212 Olive Green 44-4-91-40 104-133-42 000100 Black 75-66-60-91 10-9-10 000216 Light Cyan 62-4-9-0 88-190-221 000101 Stiff Black 75-66-60-91 10-9-10 000217 Green Gold Stringer 11-6-83-13 206-194-55 000102 Blue Black 76-69-64-85 6-7-13 000220 Sunflower Yellow 5-33-99-1 240-174-0 000104 Glacier Blue 38-3-5-0 162-211-235 000221 Citronelle 35-15-95-1 179-184-43 000108 Powder Blue 41-15-11-3 153-186-207 000222 Avocado Green 57-24-100-2 125-155-48 000112 Mint Green 43-2-49-2 155-201-152 000224 Deep Red 16-99-73-38 140-24-38 000113 White 5-2-5-0 244-245-241 000225 Pimento Red 1-100-99-11 208-10-13 000114 Cobalt Blue 86-61-0-0 43-96-170 000227 Golden Green 2-24-97-34 177-141-0 000116 Turquoise Blue 56-0-21-1 109-197-203 000236 Slate Gray 57-47-38-40 86-88-97 000117 Mineral Green 62-9-64-27 80-139-96 000241 Moss Green 66-45-98-40 73-84-36 000118 Periwinkle 66-46-1-0 102-127-188 000243 Translucent White 5-4-4-1 241-240-240 000119 Mink 37-44-37-28 132-113-113 000301 Pink 13-75-22-10 -

Spyder Amber/White Front Runner™ Installation Instructions
Spyder Amber/White Front Runner™ Installation Instructions We thank you for purchasing the Custom Dynamics® Spyder Amber/White Front Runner! Our products utilize the latest technology and high quality components to ensure you the brightest, most reliable LED’s on the market. We offer one of the best warranty programs in the industry and we back our products with excel- lent customer support, if you have questions before or during installation of this product please call Custom Dynamics® at 1(800) 382-1388. Posi-Lock™ Instructions: Package Includes: - Amber/White Front Runner™ - Amber/White Harness (pair) - 3M Promoter Pack (1) - Black Tie Wraps Instructions: 1. Test fit area to determine where Front Runner™ will be mounted. Measure the bottom lip of the front spoiler with a measuring tape to find the center. Mark center on spoiler, then align the center white line on the Front Runner™ and measure out to each end and mark that location. 2. Clean the area of the spoiler where the front runner will mount with the 3M Promoter Pad. Let Dry for 45-60 Seconds before going to the next step. 3. Remove an inch or so of the red backing from the 3M tape and adhere Front Runner™ to the edge marking on the spoiler made in step one. Double check your center mark before removing more of the tape. If alignment is good, slowly remove the rest of the red backing in small increments, applying pressure to make sure tape makes solid contact with the surface until entire Front Runner is attached firmly. -
![Attachment G – CHP AMBER, SILVER, BLUE and YELLOW Alerts [Source: CHP]](https://docslib.b-cdn.net/cover/3435/attachment-g-chp-amber-silver-blue-and-yellow-alerts-source-chp-783435.webp)
Attachment G – CHP AMBER, SILVER, BLUE and YELLOW Alerts [Source: CHP]
Attachment G – CHP AMBER, SILVER, BLUE AND YELLOW Alerts [source: CHP] By legislation the California Highway Patrol (CHP) is responsible for public alerting in specified circumstances. CHP uses a variety of alert dissemination tools including Changeable Message Signs on freeways, the broadcast Emergency Alert System (EAS), Wireless Emergency Alerts (WEA) and social media. Details appear in the following CHP Information Bulletins. G-1 INFORMATION for Allied BULLETIN Agencies BULLETIN NUMBER 217 AMBER ALERT PROGRAM UPDATE AND ENDANGERED MISSING ADVISORIES The purpose of this Information Bulletin is to provide an AMBER Alert program update and discuss the use of an Endangered Missing Advisory (EMA) for circumstances where the statutory criteria for an AMBER Alert are not met. Both procedures have proven to be very successful in locating abducted and missing children when implemented in an appropriate manner. Background America’s Missing: Broadcast Emergency Response Alert, or AMBER Alert, is a program that partners California’s law enforcement community, media broadcasting agencies, and the public in locating abducted children. The goal of an AMBER Alert is to provide the public with immediate information about a child abduction via widespread media broadcasts and to solicit help from the public in the safe and swift return of the child. In cases where the AMBER Alert criteria are not met, there are other options for law enforcement agencies to use to disseminate information to the public. Activating an AMBER Alert Local law enforcement agencies investigate reports of an abducted or missing child. Ultimately, the local investigating law enforcement agency determines if the AMBER Alert activation criteria specified in Section 8594 of the Government Code (GC) have been met. -

Levafix® Amber CA-N & Red CA-N
Sustainability is our responsibility. At DyStar, our products and services help customers worldwide reduce costs, shorten lead times and meet stringent quality and ecological specifications. Frankfurt Ludwigshafen Marcq en Baroeul Milan Kyunggi-do Porto Istanbul Reidsville, NC Mem Martins Barcelona Osaka Corlu Wuxi Charlotte, NC Nanjing Omuta Cairo Ankleshwar Shanghai Karachi Hangzhou Naucalpan Taipei Mexico City Navi Mumbai Dhaka Hong Kong ® Samutprakarn Levafix Amber CA-N & Red CA-N Bangladesh Singapore DyStar Singapore Pte. Ltd. Jakarta Perfect partner for ternary shades (Liaison Office) Gabus House No. 257 (2nd Floor, B2) São Paulo Navana Ville Akbar, Road No :19/A Apiúna New DOHS, Mohakhali, Dhaka-1206 Pietermaritzburg Tel: + 88 02 875 16 40 Fax: + 88 02 875 16 41 Global Headquarters [email protected] DyStar Office India Key Production Site Brazil DyStar India Pvt. Ltd. Agencies in 50 other countries DyStar Indústria e Comércio Plot No. R-855 de Produtos Químicos Ltda. TTC Industrial Area Av. Eng° Luis Carlos Berrini, 1645 Rabale, P.O. Ghansoli 11° andar, Cidade Monções Navi Mumbai - 400 701 Pakistan CEP 04571-011. São Paulo / SP Tel: +91 22 61 41 90 00 DyStar Pakistan (Pvt) Ltd. Taiwan Tel: +55 11 55 08 36 84 Fax: +91 22 61 41 90 10 217, Dehli Mercantile Co-operative DyStar Taiwan Ltd. Fax: +55 11 55 08 36 23 [email protected] Housing Society, Sirajudulla Road 5th Fl., No. 196, Sec. 2 [email protected] Karachi – 74800 Chien Kuo N. Road, 104 Taipei Indonesia Tel: +92 21 34 55 69 95-7 Tel: +886 2 25 16 37 77 China/Hong Kong PT DyStar Colours Indonesia Fax: +92 21 34 55 57 58 Fax: + 886 2 25 16 81 99 DyStar China Ltd. -

1018Ai Ele788 (18) 12W Rgbwai Led
1018AI ELE788 (18) 12W RGBWAI LED GENERAL INFORMATION A powerful, well-rounded stage light, the elektraLite 1018AI is ready to bring rich vibrant color at a professional level. At the heart of the elektraLite 1018AI are eighteen powerful 12-watt 6-in-1 LED’s, consisting of a red, green, blue, white, amber, and indigo component. The 6-in-1 LED uses diffraction lensing, eliminating “rainbow” color spots which are typical when individual LED’s are exposed in lighting a cyc or similar background. The elektraLite 1018AI is designed to deliver rich, vibrant colors you expect from an LED fixture, while still providing d true, brilliant, tunable white. By mixing color internally, the 1018AI’s sophisticated diffraction lensing system provide true, blended, single-color output. Because of this, the white is tunable with the amber and white LED components, and the color range for pastel and deep blues and purples is intensified with the indigo component. By adding amber and indigo to the LED emitter, the elektraLite 1018AI is ideal for a wide variety of public spaces FEATURES including stages, classrooms, galleries, worship spaces, studios, and event spaces. - LED technology; utilizing eighteen 12-watt 6-IN-1 LED’s - rich, vivid red, blue, green and white, with added amber for A user-friendly onboard control interface allows for multiple true white color toning, and indigo for pastel blending and control options including master/slave, DMX, including RGB, well-rounded color saturation strobe, and console-free static operation. - crisp 5,600K white light and balancing from 2,700K utilizing The 1018AI has 5-pin XLR DMX connectors (both in and amber thru). -

Uplighting Setup Instructions More Color Options Special Programming
UPLIGHTING SETUP INSTRUCTIONS **Keep all packaging materials. Uplights must be returned in same box / packaging.** SELECT COLOR 1. Use the power cord provided to plug the uplight into a power outlet. 2. Press “MENU” repeatedly until “C” shows on the display. 3. Press “ENTER” 4. Use the “UP” or “DOWN” button to select one of the standard color options: C1 = Red C3 = Blue C5 = Magenta C7 = White C2 = Green C4: =Light Blue C6 = Yellow **For More Colors, See Below 5. Press “ENTER” to save the color. SETUP LIGHTS 1. Place the uplight on the floor, close to a wall or other surface. 2. Tilt light towards wall until desired effect is achieved. Adjust legs by twisting knobs on each side. 3. Link uplights together. Power cords can plug into outlet OR another uplight. Link 40 lights per outlet. MORE COLOR OPTIONS To program a custom color, find your color’s “rgb” code from the chart on the back of this page. Then follow these steps: 1. Press “MENU” repeatedly until “U” shows on the display. Press “ENTER” 2. You should see “r” with a number. Press “UP” or “DOWN” to set the “r” value for your color. Press “ENTER” 3. You should see “g” with a number. Press “UP” or “DOWN” to set your “g” value. Press “ENTER” 4. You should see “b” with a number. Press “UP” or “DOWN” to set your “b” value. Press ENTER. You’re Done! SPECIAL PROGRAMMING Sound Active Mode 1. Press the MENU button repeatedly until “P—“ shows on the display. Press ENTER. 2. -

Gel List
GELS / HUE COLOR MODE GEL LIST - Numerical Active if Green / Magenta < 3 DMX VALUE GEL DMX VALUE GEL DMX VALUE GEL DMX VALUE GEL 4 KF Candle Flame 62 Fire 120 Leaf Green 178 Pale Violet 6 KF Flo Warm White 64 Sunset Red 122 Fern Green 180 Just Blue 8 KF Flo Cool White 66 Pale Gold 124 Primary Green 182 Violet 10 KF LP Sodium Vpr 68 English Rose 126 Moss Green 184 Dark Blue 12 KF Mercury Vpr 70 Bastard Amber 128 Dark Y Green 186 Surprise Pink 14 KF 20K Blue Sky 72 Surprise Peach 130 Dark Green 188 Deeper Blue 16 KF Green Screen 74 Deep Gld Amber 132 Marine Blue 190 Special M Blue 18 KF Blue Screen 76 Dark Amber 134 Peacock Blue 192 Zenith Blue 20 Smokey Pink 78 Apricot 136 Med Blue-Green 194 Light Lavender 22 Dark Magenta 80 Gold Amber 138 Sp Steel Blue 196 Palace Blue 24 Bright Rose 82 Cosmetic Peach 140 Lagoon Blue 198 Lilac Tint 26 Pale Salmon 84 Deep Orange 142 Steel Blue 200 Deep Blue 28 Light Rose 86 Chocolate 144 Lighter Blue 202 Lavender Tint 30 Light Salmon 88 Orange 146 Summer Blue 204 Pale Lavender 32 Magenta 90 White Flame 148 Light Blue 206 Dark Lavender 34 Pink 92 Medium Amber 150 Moonlight Blue 208 Lavender 36 Rosy Amber 94 Pale Amber Gold 152 Pale Blue 210 Deep Lavender 38 Pale Red 96 Chrome Orange 154 Pale Navy Blue 212 Congo Blue 40 Scarlet 98 Straw Tint 156 Mist Blue 214 Rose Purple 42 Loving Amber 100 Straw 158 Bright Blue 216 Mauve 44 Pale Rose 102 Deep Straw 160 True Blue 218 Rose Pink 46 Medium Red 104 Deep Amber 162 Dark Steel Blue 220 Follies Pink 48 Gold Tint 106 Light Amber 164 Daylight Blue 222 Bright Pink 50 -

Color Theory Guide
Contents The Three Dimensions of Color ........................................................................................ 2 Using the Three Dimensions of Color .......................................................................... 2 Special Effects, Pearls and Metallics ................................................................................. 3 Color Shifts and Tri-Coats ................................................................................................. 3 When Colors Flop ............................................................................................................. 4 Techniques for Adjusting Color ......................................................................................... 4 Application .................................................................................................................. 5 Equipment ................................................................................................................... 5 Shop Conditions .......................................................................................................... 5 Solvent ........................................................................................................................ 5 Page 1 Color Theory The Three Dimensions of Color Value, hue and chroma are standard terms used in the color industry to describe the three dimensions of color. Understanding value, hue and chroma is necessary for successful color adjustment. Please refer to the following graphic of the Color Sphere when -

An Overview of Developmental Stages of Consciousness
An Overview of Developmental Stages of Consciousness Compiled by Barrett C. Brown, Integral Institute April 3, 2006 Based upon research by: Ken Wilber in Integral theory and Integral psychology Clare Graves, Don Beck, and Chris Cowan in the development of values Jane Loevinger and Susanne Cook-Greuter in the development of self-identity Developmental psychologists have identified numerous features of an individual’s consciousness, such as cognition (what one is aware of), values (what one considers most important), and self-identity (what one identifies with).1 These features of consciousness develop through recognizable stages, each stage revealing a markedly different understanding of the world.2 Described below are eight stages of consciousness as understood through research about the unfolding development of values and self-identity.3 There are other developmental lines besides the values and self-identity lines selected here; however, these are two of the most important. Each stage of consciousness is identified with a color, for easy reference.4 It is important to recognize that these “stages” are not strict levels, like rungs on a ladder. They are more like loosely delineated areas along a spectrum of development. Thus, a stage is more like a probability wave than a concrete level of consciousness. The stages below are divided into egocentric stages (Infrared, Magenta, and Red), ethnocentric (Amber), worldcentric (Orange and Green), and kosmocentric stages (Teal, Turquoise, and Indigo). Those interested in learning more are encouraged to review the original sources listed in the references. 1 Wilber, 2000a, 2005 2 e.g., Wilber 2000a, Beck & Cowan, 1996; Graves, 2005; Kegan, 1982; Loevinger, 1976 3 I have compiled this information from many sources; the publicly available material I drew from is referenced.