Indigo Carmine Stop-’N-Go Light SCIENTIFIC
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Title == Red/Amber Report
DOT HS 811 115 April 2009 The Effectiveness of Amber Rear Turn Signals for Reducing Rear Impacts This report is free of charge from the NHTSA Web site at www.nhtsa.dot.gov This publication is distributed by the U.S. Department of Transportation, National Highway Traffic Safety Administration, in the interest of information exchange. The opinions, findings, and conclusions expressed in this publication are those of the author and not necessarily those of the Department of Transportation or the National Highway Traffic Safety Administration. The United States Government assumes no liability for its content or use thereof. If trade or manufacturer’s names or products are mentioned, it is because they are considered essential to the object of the publication and should not be construed as an endorsement. The United States Government does not endorse products or manufacturers. Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient’s Catalog No. DOT HS 811 115 4. Title and Subtitle 5. Report Date The Effectiveness of Amber Rear Turn Signals for Reducing Rear April 2009 Impacts 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Kirk Allen, Ph.D. 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Evaluation Division; National Center for Statistics and Analysis National Highway Traffic Safety Administration 11. Contract or Grant No. Washington, DC 20590 12. Sponsoring Agency Name and Address 13. Type of Report and Period Covered National Highway Traffic Safety Administration NHTSA Technical Report 1200 New Jersey Avenue SE. 14. Sponsoring Agency Code Washington, DC 20590 15. -

Mid Atlantic Carnival Glass Jamboree Thursday October 17 and Saturday October 19, 2019
Mid Atlantic Carnival Glass Jamboree Thursday October 17 and Saturday October 19, 2019 Thursday Day One; Lots 1-175 ____1. Fenton Contemporary Red Diamond Rib Basket. ____29. NORTHWOOD BLUE POPPY SHOW PLATE. ____2. 1987 ACGA Green Morning Glory Pitcher Souvenir. (NICE EXAMPLE.) ____3. Pair of 1998 & 2000 HOACGA Room Display Awards. ____30. NORTHWOOD GREEN POPPY SHOW PLATE. (Clear Opal and Blue Hobnail Spittoons.) (VERY NICE EXAMPLE.) ____4. 1991 & 1996 ACGA Red Inverted Strawberry ____31. Northwood Purple Strawberry Plate. (Has Basketweave Souvenirs. (Handled Basket and Creamer.) Exterior and Good Iridescence.) ____5. 2001 ACGA Vaseline Opal Seacoast Spittoon Souvenir. ____32. Northwood Blue Three Fruits Stippled PCE Bowl. ____6. (3) ACGA Green Opal Seacoast Spittoon Souvenirs. (Ribbed Exterior. Nice Iridescence.) (Columbus Ohio.) ____33. Northwood Green Greek Key PCE Bowl. ____7. Fenton Contemporary Bulbous Vase. (Tri-Cornered (Has Basketweave Exterior.) Crimped Edge.) ____34. Northwood Purple Good Luck Ruffled Bowl. ____8. 1998 & 1999 HOACGA Souvenirs. (White Loving Cup (Ribbed Exterior.) and Vaseline Opal Fine Cut & Roses Rosebowl.) ____35. NORTHWOOD GREEN PEACOCK AT THE URN ____9. Imperial Electric Purple Water Carafe & Wine Decanter. MASTER ICS BOWL. (VERY NICE EXAMPLE; RARE (Decanter Has No Stopper.) COLOR.) ____10. IMPERIAL ELECTRIC PURPLE BROKEN ARCHES ____36. NORTHWOOD BLUE PEACOCK AT THE URN PUNCH BOWL. (GORGEOUS IRIDESCENCE AND MASTER ICS BOWL. (NICE ELECTRIC PRETTY DARN EVEN.) HIGHLIGHTS.) ____11. (3) Imperial Purple Broken Arches Punch Cups. ____37. Northwood Amethyst Peacock at the Urn Master ICS ____12. Imperial Electric Purple Diamond Lace 5 PC. Water Set. Bowl. (One Tumbler Has Edge Nick as Seen in Photos.) ____38. Northwood Dark Marigold Peacock at the Urn Master ____13. -

Elektrabar Mini (8) 12W RGBWAI LED Linear Strip
ELE815 ElektraBar Mini (8) 12W RGBWAI LED Linear Strip GENERAL INFORMATION A powerful, full‐featured linear strip fixture, the elektraLite ElektraBar Mini is ready to bring rich vibrant color at a professional level. At the heart of the elektraLite ElektraBar Mini are eight powerful 12‐watt 6‐in‐1 LED’s, consisting of a red, green, blue, white, amber, and indigo component. The elektraBar Mini features individual control of each pixel, allowing for 16 million colors on eight different LED pixels. With precision spacing, the fixture is also ideal for low‐resolution pixel mapping and video wall applications. The elektraLite elektraBar Mini is designed to deliver rich, vibrant colors you expect from an LED fixture, while still d providing true, brilliant, tunable white. By mixing color internally, the ElektraBar Mini’s sophisticated diffraction lensing system provide true, blended, single‐color output. Because of this, the color range for pastel and deep blues and purples is intensified with the indigo component. FEATURES ORDERING INFORMATION ELE815 ElektraBar Mini Linear Strip 25° (black) ‐ utilizes eight individually controllable 12‐watt 6‐IN‐1 LED’s ELE815‐10 10° Lens Kit (set of 8) ‐ rich, vivid red, blue, green and white, with added amber for ELE815‐40 40° Lens Kit (set of 8) true white color toning, and indigo for pastel blending and ELE815‐GS 4” adjustable Glare Shield / Beam Flange well‐rounded color saturation ELE724‐AP* 12” Edison to IP68 Power Adapter ‐ individual control of each pixel, allowing 16 million colors ELE724‐AD* 12” -
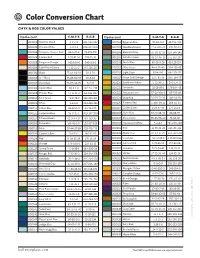
Color Conversion Chart
Color Conversion Chart CMYK & RGB COLOR VALUES Opalescent C-M-Y-K R-G-B Opalescent C-M-Y-K R-G-B 000009 Reactive Cloud 4-2-1-0 241-243-247 000164 Egyptian Blue 81-48-0-0 49-116-184 000013 Opaque White 4-2-2-1 246-247-249 000203 Woodland Brown 22-63-87-49 120-70-29 000016 Turquoise Opaque Rod 65-4-27-6 75-174-179 000206 Elephant Gray 35-30-32-18 150-145-142 000024 Tomato Red 1-99-81-16 198-15-36 000207 Celadon Green 43-14-46-13 141-167-137 000025 Tangerine Orange 1-63-100-0 240-119-2 000208 Dusty Blue 60-25-9-28 83-123-154 000034 Light Peach Cream 5-12-15-0 243-226-213 000212 Olive Green 44-4-91-40 104-133-42 000100 Black 75-66-60-91 10-9-10 000216 Light Cyan 62-4-9-0 88-190-221 000101 Stiff Black 75-66-60-91 10-9-10 000217 Green Gold Stringer 11-6-83-13 206-194-55 000102 Blue Black 76-69-64-85 6-7-13 000220 Sunflower Yellow 5-33-99-1 240-174-0 000104 Glacier Blue 38-3-5-0 162-211-235 000221 Citronelle 35-15-95-1 179-184-43 000108 Powder Blue 41-15-11-3 153-186-207 000222 Avocado Green 57-24-100-2 125-155-48 000112 Mint Green 43-2-49-2 155-201-152 000224 Deep Red 16-99-73-38 140-24-38 000113 White 5-2-5-0 244-245-241 000225 Pimento Red 1-100-99-11 208-10-13 000114 Cobalt Blue 86-61-0-0 43-96-170 000227 Golden Green 2-24-97-34 177-141-0 000116 Turquoise Blue 56-0-21-1 109-197-203 000236 Slate Gray 57-47-38-40 86-88-97 000117 Mineral Green 62-9-64-27 80-139-96 000241 Moss Green 66-45-98-40 73-84-36 000118 Periwinkle 66-46-1-0 102-127-188 000243 Translucent White 5-4-4-1 241-240-240 000119 Mink 37-44-37-28 132-113-113 000301 Pink 13-75-22-10 -

Use of Natural Colours in the Ice Cream Industry
USE OF NATURAL COLOURS IN THE ICE CREAM INDUSTRY Dr. Juan Mario Sanz Penella Dr. Emanuele Pedrazzini Dr. José García Reverter SECNA NATURAL INGREDIENTS GROUP Definition of ice cream Ice cream is a frozen dessert, a term that includes different types of product that are consumed frozen and that includes sorbets, frozen yogurts, non-dairy frozen desserts and, of course, ice cream. In order to simplify its classification, we can consider for practical purposes two different types of frozen desserts: ice cream and sorbets. Ice cream includes all products that have a neutral pH and contain dairy ingredients. While sorbet refers to products with an acidic pH, made with water and other ingredients such as fruit, but without the use of dairy. Additionally, when referring to ice cream, we are not referring to a single type of product, we are actually referring to a wide range of these. Ice cream is a food in which the three states of matter coexist, namely: water in liquid form and as ice crystals, sugars, fats and proteins in solid form and occluded air bubbles in gaseous form, Figure 1. Artisanal ice creams based on natural ingredients which give it its special texture. It should be noted that ice cream is a very complete food from a nutritional point of view since it incorporates in its formulation a wide range of ingredients (sugars, milk, fruits, egg products ...) essential for a balanced diet. Another aspect of great relevance in the manufacture of ice cream is the hedonic factor. Ice cream is consumed mainly for the pure pleasure of tasting it, the hedonic component being the main factor that triggers its consumption, considering its formulation and design a series of strategies to evoke a full range of sensations: gustatory, olfactory, visual and even, of the touch and the ear. -

Spyder Amber/White Front Runner™ Installation Instructions
Spyder Amber/White Front Runner™ Installation Instructions We thank you for purchasing the Custom Dynamics® Spyder Amber/White Front Runner! Our products utilize the latest technology and high quality components to ensure you the brightest, most reliable LED’s on the market. We offer one of the best warranty programs in the industry and we back our products with excel- lent customer support, if you have questions before or during installation of this product please call Custom Dynamics® at 1(800) 382-1388. Posi-Lock™ Instructions: Package Includes: - Amber/White Front Runner™ - Amber/White Harness (pair) - 3M Promoter Pack (1) - Black Tie Wraps Instructions: 1. Test fit area to determine where Front Runner™ will be mounted. Measure the bottom lip of the front spoiler with a measuring tape to find the center. Mark center on spoiler, then align the center white line on the Front Runner™ and measure out to each end and mark that location. 2. Clean the area of the spoiler where the front runner will mount with the 3M Promoter Pad. Let Dry for 45-60 Seconds before going to the next step. 3. Remove an inch or so of the red backing from the 3M tape and adhere Front Runner™ to the edge marking on the spoiler made in step one. Double check your center mark before removing more of the tape. If alignment is good, slowly remove the rest of the red backing in small increments, applying pressure to make sure tape makes solid contact with the surface until entire Front Runner is attached firmly. -

Global Regulations of Food Colors Each Region Has Its Own Definitions of What Constitutes a Color Additive, with Related Use Requirements and Restrictions
Global Regulations of Food Colors Each region has its own definitions of what constitutes a color additive, with related use requirements and restrictions. Sue Ann McAvoy Sensient Colors LLC t is said, we eat with our eyes . Since antiq - food colors was soon recognized as a threat Iuity, humans have used the color of a to public health. Of concern was that some food to discern its quality. Color provides of the substances were known to be poi - a way to judge ripeness, perceive flavor and sonous and were often incorporated to hide assess quality of food. poor quality, add bulk to foods and to pass Ancient civilizations introduced color off imitation foods as real. into their foods. The ancient Egyptians col - On February 1, 1899, the executive com - ored their food yellow with saffron, and the mittee of the National Confectioners’ Asso - ancient Mayans used annatto to color their ciation published an official circular which Sue Ann McAvoy is food orange-red. Wealthy Romans ate bread was “to throw light upon the vexed question global regulatory scien - that had been whitened by adding alum to of what colors may be safely used in confec - tist for Sensient Food the flour. Color could be used to enhance tionery” as “there may at times be a doubt in Colors LLC. She has worked at Sensient the mind of the honest confectioner as to the physical appearance of the product. since 1979. However, if it made the food appear to be which colors, flavors, or ingredients he may of better quality than it was, that was con - safely use and which he may reject.” This list sidered a deceitful practice. -

Natural Colour Book
THE COLOUR BOOK Sensient Food Colors Europe INDEX NATURAL COLOURS AND COLOURING FOODS INDEX 46 Lycopene 4 We Brighten Your World 47 Antho Blends – Pink Shade 6 Naturally Different 48 Red Cabbage 8 The Colour of Innovation 49 Beetroot – with reduced bluish tone 10 Natural Colours, Colouring Foods 50 Beetroot 11 Cardea™, Pure-S™ 51 Black Carrot 12 YELLOW 52 Grape 14 Colourful Impulses 53 Enocianin 15 Carthamus 54 Red Blends 16 Curcumin 56 VIOLET & BLUE 17 Riboflavin 59 Violet Blends 18 Lutein 61 Spirulina 19 Carrot 62 GREEN 20 Natural Carotene 65 Green Blends 22 Beta-Carotene 66 Copper-Chlorophyllin 24 Annatto 67 Copper-Chlorophyll 25 Yellow/ Orange Blends 68 Chlorophyll/-in 26 ORANGE 69 Spinach 29 Natural Carotene 70 BROWN 30 Paprika Extract 73 Burnt Sugar 32 Carrot 74 Apple 33 Apocarotenal 75 Caramel 34 Carminic Acid 76 BLACK & WHITE 35 Beta-Carotene 79 Vegetable Carbon 36 RED 80 Titanium Dioxide 39 Antho Blends – Strawberry Shade 81 Natural White 40 Aronia 41 Elderberry 83 Regulatory Information 42 Black Carrot 84 Disclaimer 43 Hibiscus 85 Contact Address 44 Carmine 3 INDEX NATURAL COLOURS AND COLOURING FOODS WE BRIGHTEN YOUR WORLD Sensient is as colourful as the world around us. Whatever you are looking for, across the whole spectrum of colour use, we can deliver colouring solutions to best meet your needs in your market. Operating in the global market place for over 100 years Sensient both promises and delivers proven international experience, expertise and capabilities in product development, supply chain management, manufacture, quality management and application excellence of innovative colours for food and beverages. -
![Attachment G – CHP AMBER, SILVER, BLUE and YELLOW Alerts [Source: CHP]](https://docslib.b-cdn.net/cover/3435/attachment-g-chp-amber-silver-blue-and-yellow-alerts-source-chp-783435.webp)
Attachment G – CHP AMBER, SILVER, BLUE and YELLOW Alerts [Source: CHP]
Attachment G – CHP AMBER, SILVER, BLUE AND YELLOW Alerts [source: CHP] By legislation the California Highway Patrol (CHP) is responsible for public alerting in specified circumstances. CHP uses a variety of alert dissemination tools including Changeable Message Signs on freeways, the broadcast Emergency Alert System (EAS), Wireless Emergency Alerts (WEA) and social media. Details appear in the following CHP Information Bulletins. G-1 INFORMATION for Allied BULLETIN Agencies BULLETIN NUMBER 217 AMBER ALERT PROGRAM UPDATE AND ENDANGERED MISSING ADVISORIES The purpose of this Information Bulletin is to provide an AMBER Alert program update and discuss the use of an Endangered Missing Advisory (EMA) for circumstances where the statutory criteria for an AMBER Alert are not met. Both procedures have proven to be very successful in locating abducted and missing children when implemented in an appropriate manner. Background America’s Missing: Broadcast Emergency Response Alert, or AMBER Alert, is a program that partners California’s law enforcement community, media broadcasting agencies, and the public in locating abducted children. The goal of an AMBER Alert is to provide the public with immediate information about a child abduction via widespread media broadcasts and to solicit help from the public in the safe and swift return of the child. In cases where the AMBER Alert criteria are not met, there are other options for law enforcement agencies to use to disseminate information to the public. Activating an AMBER Alert Local law enforcement agencies investigate reports of an abducted or missing child. Ultimately, the local investigating law enforcement agency determines if the AMBER Alert activation criteria specified in Section 8594 of the Government Code (GC) have been met. -

Levafix® Amber CA-N & Red CA-N
Sustainability is our responsibility. At DyStar, our products and services help customers worldwide reduce costs, shorten lead times and meet stringent quality and ecological specifications. Frankfurt Ludwigshafen Marcq en Baroeul Milan Kyunggi-do Porto Istanbul Reidsville, NC Mem Martins Barcelona Osaka Corlu Wuxi Charlotte, NC Nanjing Omuta Cairo Ankleshwar Shanghai Karachi Hangzhou Naucalpan Taipei Mexico City Navi Mumbai Dhaka Hong Kong ® Samutprakarn Levafix Amber CA-N & Red CA-N Bangladesh Singapore DyStar Singapore Pte. Ltd. Jakarta Perfect partner for ternary shades (Liaison Office) Gabus House No. 257 (2nd Floor, B2) São Paulo Navana Ville Akbar, Road No :19/A Apiúna New DOHS, Mohakhali, Dhaka-1206 Pietermaritzburg Tel: + 88 02 875 16 40 Fax: + 88 02 875 16 41 Global Headquarters [email protected] DyStar Office India Key Production Site Brazil DyStar India Pvt. Ltd. Agencies in 50 other countries DyStar Indústria e Comércio Plot No. R-855 de Produtos Químicos Ltda. TTC Industrial Area Av. Eng° Luis Carlos Berrini, 1645 Rabale, P.O. Ghansoli 11° andar, Cidade Monções Navi Mumbai - 400 701 Pakistan CEP 04571-011. São Paulo / SP Tel: +91 22 61 41 90 00 DyStar Pakistan (Pvt) Ltd. Taiwan Tel: +55 11 55 08 36 84 Fax: +91 22 61 41 90 10 217, Dehli Mercantile Co-operative DyStar Taiwan Ltd. Fax: +55 11 55 08 36 23 [email protected] Housing Society, Sirajudulla Road 5th Fl., No. 196, Sec. 2 [email protected] Karachi – 74800 Chien Kuo N. Road, 104 Taipei Indonesia Tel: +92 21 34 55 69 95-7 Tel: +886 2 25 16 37 77 China/Hong Kong PT DyStar Colours Indonesia Fax: +92 21 34 55 57 58 Fax: + 886 2 25 16 81 99 DyStar China Ltd. -

Fuchsias List
Fuchsias List All £2.00 Each, listed by alphabetical order within categories. Numbers 1 - 47 : Trailing Fuchsias Numbers 48 - 63 : Upright Fuchsias Numbers 64 - 78 : Hardy Fuchsias Trailing Fuchsias All £2.00 Each 1) Annabelle - White Slight Flushed Pink 2) Auntie Jinks - Cerise Purple + White 3) Adrienne - Violet + Rose Red 4) Anthea- Lilac Purple + White 5) Ballet Girl - White + Red 6) Bella Rosella - Bright Pink + Light Pink 7) Bicentennial - Cerise Flushed Orange 8) Blue Eyes - Violet Blue + Red 9) Blue Mirage - Pinky Blue + White 10) Blue Sarah - Lilac Blue + White 11) Claudia - Light Soft Pink 12) Coachman - Rich Orange + Pale Salmon 13) Cecile - Lilac + Rose Pink 14) Deep Purple - Deep Purple + White 15) Dorothy Clive - Aubergine + Red 16) Dancing Flame - Carmine Orange + Pale Orange 17) Dark Eyes - Violet + Deep Red 18) Dawn Star - Lavender + Pale Rose 19) Eva Boerg - Purple + Rose Pink 20) Golden Swingtime - Red + White + Variegated 21) Harry Grey - White + Rose Pink Shading 22) Jean Taylor - Lavender + Scarlet 23) Jean Lilley - Pale Pink + Deep Rose 24) Kit Oxtoby - Rose Pink + Rose Pink 25) Lauren - Light Rose Pink 26) La Campanella - Purple + White Flushed Pink 27) La Campanella Pink - Magenta + Pale Carmine 28) Marinka - Dark Red + Red 29) Patricia Hodge - Salmon Pink + Tangerine 30) Purple Fountain - Purple + Red 31) Pink Galore - Soft Rose Pink 32) Peachy - Peachy Pink 33) Pink Marshmallow - Pinky White 34) Quasar - Lilac Blue + White 35) Red Spider - Deep Crimson + Rose 36) Rose of Denmark - Rosy Purple + White 37) Sir -

1018Ai Ele788 (18) 12W Rgbwai Led
1018AI ELE788 (18) 12W RGBWAI LED GENERAL INFORMATION A powerful, well-rounded stage light, the elektraLite 1018AI is ready to bring rich vibrant color at a professional level. At the heart of the elektraLite 1018AI are eighteen powerful 12-watt 6-in-1 LED’s, consisting of a red, green, blue, white, amber, and indigo component. The 6-in-1 LED uses diffraction lensing, eliminating “rainbow” color spots which are typical when individual LED’s are exposed in lighting a cyc or similar background. The elektraLite 1018AI is designed to deliver rich, vibrant colors you expect from an LED fixture, while still providing d true, brilliant, tunable white. By mixing color internally, the 1018AI’s sophisticated diffraction lensing system provide true, blended, single-color output. Because of this, the white is tunable with the amber and white LED components, and the color range for pastel and deep blues and purples is intensified with the indigo component. By adding amber and indigo to the LED emitter, the elektraLite 1018AI is ideal for a wide variety of public spaces FEATURES including stages, classrooms, galleries, worship spaces, studios, and event spaces. - LED technology; utilizing eighteen 12-watt 6-IN-1 LED’s - rich, vivid red, blue, green and white, with added amber for A user-friendly onboard control interface allows for multiple true white color toning, and indigo for pastel blending and control options including master/slave, DMX, including RGB, well-rounded color saturation strobe, and console-free static operation. - crisp 5,600K white light and balancing from 2,700K utilizing The 1018AI has 5-pin XLR DMX connectors (both in and amber thru).