Identification and Care of Amber
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Title == Red/Amber Report
DOT HS 811 115 April 2009 The Effectiveness of Amber Rear Turn Signals for Reducing Rear Impacts This report is free of charge from the NHTSA Web site at www.nhtsa.dot.gov This publication is distributed by the U.S. Department of Transportation, National Highway Traffic Safety Administration, in the interest of information exchange. The opinions, findings, and conclusions expressed in this publication are those of the author and not necessarily those of the Department of Transportation or the National Highway Traffic Safety Administration. The United States Government assumes no liability for its content or use thereof. If trade or manufacturer’s names or products are mentioned, it is because they are considered essential to the object of the publication and should not be construed as an endorsement. The United States Government does not endorse products or manufacturers. Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient’s Catalog No. DOT HS 811 115 4. Title and Subtitle 5. Report Date The Effectiveness of Amber Rear Turn Signals for Reducing Rear April 2009 Impacts 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Kirk Allen, Ph.D. 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Evaluation Division; National Center for Statistics and Analysis National Highway Traffic Safety Administration 11. Contract or Grant No. Washington, DC 20590 12. Sponsoring Agency Name and Address 13. Type of Report and Period Covered National Highway Traffic Safety Administration NHTSA Technical Report 1200 New Jersey Avenue SE. 14. Sponsoring Agency Code Washington, DC 20590 15. -

Mid Atlantic Carnival Glass Jamboree Thursday October 17 and Saturday October 19, 2019
Mid Atlantic Carnival Glass Jamboree Thursday October 17 and Saturday October 19, 2019 Thursday Day One; Lots 1-175 ____1. Fenton Contemporary Red Diamond Rib Basket. ____29. NORTHWOOD BLUE POPPY SHOW PLATE. ____2. 1987 ACGA Green Morning Glory Pitcher Souvenir. (NICE EXAMPLE.) ____3. Pair of 1998 & 2000 HOACGA Room Display Awards. ____30. NORTHWOOD GREEN POPPY SHOW PLATE. (Clear Opal and Blue Hobnail Spittoons.) (VERY NICE EXAMPLE.) ____4. 1991 & 1996 ACGA Red Inverted Strawberry ____31. Northwood Purple Strawberry Plate. (Has Basketweave Souvenirs. (Handled Basket and Creamer.) Exterior and Good Iridescence.) ____5. 2001 ACGA Vaseline Opal Seacoast Spittoon Souvenir. ____32. Northwood Blue Three Fruits Stippled PCE Bowl. ____6. (3) ACGA Green Opal Seacoast Spittoon Souvenirs. (Ribbed Exterior. Nice Iridescence.) (Columbus Ohio.) ____33. Northwood Green Greek Key PCE Bowl. ____7. Fenton Contemporary Bulbous Vase. (Tri-Cornered (Has Basketweave Exterior.) Crimped Edge.) ____34. Northwood Purple Good Luck Ruffled Bowl. ____8. 1998 & 1999 HOACGA Souvenirs. (White Loving Cup (Ribbed Exterior.) and Vaseline Opal Fine Cut & Roses Rosebowl.) ____35. NORTHWOOD GREEN PEACOCK AT THE URN ____9. Imperial Electric Purple Water Carafe & Wine Decanter. MASTER ICS BOWL. (VERY NICE EXAMPLE; RARE (Decanter Has No Stopper.) COLOR.) ____10. IMPERIAL ELECTRIC PURPLE BROKEN ARCHES ____36. NORTHWOOD BLUE PEACOCK AT THE URN PUNCH BOWL. (GORGEOUS IRIDESCENCE AND MASTER ICS BOWL. (NICE ELECTRIC PRETTY DARN EVEN.) HIGHLIGHTS.) ____11. (3) Imperial Purple Broken Arches Punch Cups. ____37. Northwood Amethyst Peacock at the Urn Master ICS ____12. Imperial Electric Purple Diamond Lace 5 PC. Water Set. Bowl. (One Tumbler Has Edge Nick as Seen in Photos.) ____38. Northwood Dark Marigold Peacock at the Urn Master ____13. -

Elektrabar Mini (8) 12W RGBWAI LED Linear Strip
ELE815 ElektraBar Mini (8) 12W RGBWAI LED Linear Strip GENERAL INFORMATION A powerful, full‐featured linear strip fixture, the elektraLite ElektraBar Mini is ready to bring rich vibrant color at a professional level. At the heart of the elektraLite ElektraBar Mini are eight powerful 12‐watt 6‐in‐1 LED’s, consisting of a red, green, blue, white, amber, and indigo component. The elektraBar Mini features individual control of each pixel, allowing for 16 million colors on eight different LED pixels. With precision spacing, the fixture is also ideal for low‐resolution pixel mapping and video wall applications. The elektraLite elektraBar Mini is designed to deliver rich, vibrant colors you expect from an LED fixture, while still d providing true, brilliant, tunable white. By mixing color internally, the ElektraBar Mini’s sophisticated diffraction lensing system provide true, blended, single‐color output. Because of this, the color range for pastel and deep blues and purples is intensified with the indigo component. FEATURES ORDERING INFORMATION ELE815 ElektraBar Mini Linear Strip 25° (black) ‐ utilizes eight individually controllable 12‐watt 6‐IN‐1 LED’s ELE815‐10 10° Lens Kit (set of 8) ‐ rich, vivid red, blue, green and white, with added amber for ELE815‐40 40° Lens Kit (set of 8) true white color toning, and indigo for pastel blending and ELE815‐GS 4” adjustable Glare Shield / Beam Flange well‐rounded color saturation ELE724‐AP* 12” Edison to IP68 Power Adapter ‐ individual control of each pixel, allowing 16 million colors ELE724‐AD* 12” -
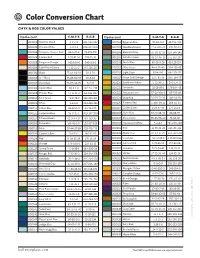
Color Conversion Chart
Color Conversion Chart CMYK & RGB COLOR VALUES Opalescent C-M-Y-K R-G-B Opalescent C-M-Y-K R-G-B 000009 Reactive Cloud 4-2-1-0 241-243-247 000164 Egyptian Blue 81-48-0-0 49-116-184 000013 Opaque White 4-2-2-1 246-247-249 000203 Woodland Brown 22-63-87-49 120-70-29 000016 Turquoise Opaque Rod 65-4-27-6 75-174-179 000206 Elephant Gray 35-30-32-18 150-145-142 000024 Tomato Red 1-99-81-16 198-15-36 000207 Celadon Green 43-14-46-13 141-167-137 000025 Tangerine Orange 1-63-100-0 240-119-2 000208 Dusty Blue 60-25-9-28 83-123-154 000034 Light Peach Cream 5-12-15-0 243-226-213 000212 Olive Green 44-4-91-40 104-133-42 000100 Black 75-66-60-91 10-9-10 000216 Light Cyan 62-4-9-0 88-190-221 000101 Stiff Black 75-66-60-91 10-9-10 000217 Green Gold Stringer 11-6-83-13 206-194-55 000102 Blue Black 76-69-64-85 6-7-13 000220 Sunflower Yellow 5-33-99-1 240-174-0 000104 Glacier Blue 38-3-5-0 162-211-235 000221 Citronelle 35-15-95-1 179-184-43 000108 Powder Blue 41-15-11-3 153-186-207 000222 Avocado Green 57-24-100-2 125-155-48 000112 Mint Green 43-2-49-2 155-201-152 000224 Deep Red 16-99-73-38 140-24-38 000113 White 5-2-5-0 244-245-241 000225 Pimento Red 1-100-99-11 208-10-13 000114 Cobalt Blue 86-61-0-0 43-96-170 000227 Golden Green 2-24-97-34 177-141-0 000116 Turquoise Blue 56-0-21-1 109-197-203 000236 Slate Gray 57-47-38-40 86-88-97 000117 Mineral Green 62-9-64-27 80-139-96 000241 Moss Green 66-45-98-40 73-84-36 000118 Periwinkle 66-46-1-0 102-127-188 000243 Translucent White 5-4-4-1 241-240-240 000119 Mink 37-44-37-28 132-113-113 000301 Pink 13-75-22-10 -

Spyder Amber/White Front Runner™ Installation Instructions
Spyder Amber/White Front Runner™ Installation Instructions We thank you for purchasing the Custom Dynamics® Spyder Amber/White Front Runner! Our products utilize the latest technology and high quality components to ensure you the brightest, most reliable LED’s on the market. We offer one of the best warranty programs in the industry and we back our products with excel- lent customer support, if you have questions before or during installation of this product please call Custom Dynamics® at 1(800) 382-1388. Posi-Lock™ Instructions: Package Includes: - Amber/White Front Runner™ - Amber/White Harness (pair) - 3M Promoter Pack (1) - Black Tie Wraps Instructions: 1. Test fit area to determine where Front Runner™ will be mounted. Measure the bottom lip of the front spoiler with a measuring tape to find the center. Mark center on spoiler, then align the center white line on the Front Runner™ and measure out to each end and mark that location. 2. Clean the area of the spoiler where the front runner will mount with the 3M Promoter Pad. Let Dry for 45-60 Seconds before going to the next step. 3. Remove an inch or so of the red backing from the 3M tape and adhere Front Runner™ to the edge marking on the spoiler made in step one. Double check your center mark before removing more of the tape. If alignment is good, slowly remove the rest of the red backing in small increments, applying pressure to make sure tape makes solid contact with the surface until entire Front Runner is attached firmly. -
![Attachment G – CHP AMBER, SILVER, BLUE and YELLOW Alerts [Source: CHP]](https://docslib.b-cdn.net/cover/3435/attachment-g-chp-amber-silver-blue-and-yellow-alerts-source-chp-783435.webp)
Attachment G – CHP AMBER, SILVER, BLUE and YELLOW Alerts [Source: CHP]
Attachment G – CHP AMBER, SILVER, BLUE AND YELLOW Alerts [source: CHP] By legislation the California Highway Patrol (CHP) is responsible for public alerting in specified circumstances. CHP uses a variety of alert dissemination tools including Changeable Message Signs on freeways, the broadcast Emergency Alert System (EAS), Wireless Emergency Alerts (WEA) and social media. Details appear in the following CHP Information Bulletins. G-1 INFORMATION for Allied BULLETIN Agencies BULLETIN NUMBER 217 AMBER ALERT PROGRAM UPDATE AND ENDANGERED MISSING ADVISORIES The purpose of this Information Bulletin is to provide an AMBER Alert program update and discuss the use of an Endangered Missing Advisory (EMA) for circumstances where the statutory criteria for an AMBER Alert are not met. Both procedures have proven to be very successful in locating abducted and missing children when implemented in an appropriate manner. Background America’s Missing: Broadcast Emergency Response Alert, or AMBER Alert, is a program that partners California’s law enforcement community, media broadcasting agencies, and the public in locating abducted children. The goal of an AMBER Alert is to provide the public with immediate information about a child abduction via widespread media broadcasts and to solicit help from the public in the safe and swift return of the child. In cases where the AMBER Alert criteria are not met, there are other options for law enforcement agencies to use to disseminate information to the public. Activating an AMBER Alert Local law enforcement agencies investigate reports of an abducted or missing child. Ultimately, the local investigating law enforcement agency determines if the AMBER Alert activation criteria specified in Section 8594 of the Government Code (GC) have been met. -

Levafix® Amber CA-N & Red CA-N
Sustainability is our responsibility. At DyStar, our products and services help customers worldwide reduce costs, shorten lead times and meet stringent quality and ecological specifications. Frankfurt Ludwigshafen Marcq en Baroeul Milan Kyunggi-do Porto Istanbul Reidsville, NC Mem Martins Barcelona Osaka Corlu Wuxi Charlotte, NC Nanjing Omuta Cairo Ankleshwar Shanghai Karachi Hangzhou Naucalpan Taipei Mexico City Navi Mumbai Dhaka Hong Kong ® Samutprakarn Levafix Amber CA-N & Red CA-N Bangladesh Singapore DyStar Singapore Pte. Ltd. Jakarta Perfect partner for ternary shades (Liaison Office) Gabus House No. 257 (2nd Floor, B2) São Paulo Navana Ville Akbar, Road No :19/A Apiúna New DOHS, Mohakhali, Dhaka-1206 Pietermaritzburg Tel: + 88 02 875 16 40 Fax: + 88 02 875 16 41 Global Headquarters [email protected] DyStar Office India Key Production Site Brazil DyStar India Pvt. Ltd. Agencies in 50 other countries DyStar Indústria e Comércio Plot No. R-855 de Produtos Químicos Ltda. TTC Industrial Area Av. Eng° Luis Carlos Berrini, 1645 Rabale, P.O. Ghansoli 11° andar, Cidade Monções Navi Mumbai - 400 701 Pakistan CEP 04571-011. São Paulo / SP Tel: +91 22 61 41 90 00 DyStar Pakistan (Pvt) Ltd. Taiwan Tel: +55 11 55 08 36 84 Fax: +91 22 61 41 90 10 217, Dehli Mercantile Co-operative DyStar Taiwan Ltd. Fax: +55 11 55 08 36 23 [email protected] Housing Society, Sirajudulla Road 5th Fl., No. 196, Sec. 2 [email protected] Karachi – 74800 Chien Kuo N. Road, 104 Taipei Indonesia Tel: +92 21 34 55 69 95-7 Tel: +886 2 25 16 37 77 China/Hong Kong PT DyStar Colours Indonesia Fax: +92 21 34 55 57 58 Fax: + 886 2 25 16 81 99 DyStar China Ltd. -

Historical Painting Techniques, Materials, and Studio Practice
Historical Painting Techniques, Materials, and Studio Practice PUBLICATIONS COORDINATION: Dinah Berland EDITING & PRODUCTION COORDINATION: Corinne Lightweaver EDITORIAL CONSULTATION: Jo Hill COVER DESIGN: Jackie Gallagher-Lange PRODUCTION & PRINTING: Allen Press, Inc., Lawrence, Kansas SYMPOSIUM ORGANIZERS: Erma Hermens, Art History Institute of the University of Leiden Marja Peek, Central Research Laboratory for Objects of Art and Science, Amsterdam © 1995 by The J. Paul Getty Trust All rights reserved Printed in the United States of America ISBN 0-89236-322-3 The Getty Conservation Institute is committed to the preservation of cultural heritage worldwide. The Institute seeks to advance scientiRc knowledge and professional practice and to raise public awareness of conservation. Through research, training, documentation, exchange of information, and ReId projects, the Institute addresses issues related to the conservation of museum objects and archival collections, archaeological monuments and sites, and historic bUildings and cities. The Institute is an operating program of the J. Paul Getty Trust. COVER ILLUSTRATION Gherardo Cibo, "Colchico," folio 17r of Herbarium, ca. 1570. Courtesy of the British Library. FRONTISPIECE Detail from Jan Baptiste Collaert, Color Olivi, 1566-1628. After Johannes Stradanus. Courtesy of the Rijksmuseum-Stichting, Amsterdam. Library of Congress Cataloguing-in-Publication Data Historical painting techniques, materials, and studio practice : preprints of a symposium [held at] University of Leiden, the Netherlands, 26-29 June 1995/ edited by Arie Wallert, Erma Hermens, and Marja Peek. p. cm. Includes bibliographical references. ISBN 0-89236-322-3 (pbk.) 1. Painting-Techniques-Congresses. 2. Artists' materials- -Congresses. 3. Polychromy-Congresses. I. Wallert, Arie, 1950- II. Hermens, Erma, 1958- . III. Peek, Marja, 1961- ND1500.H57 1995 751' .09-dc20 95-9805 CIP Second printing 1996 iv Contents vii Foreword viii Preface 1 Leslie A. -

“Among the Most Unusual Substances on Earth, Amber Combines Alluring Physical Properties with an Unparalleled Archive of Ancient Life on Our Planet
(a.) (b.) Figure 1. a) Termites in Colombian copal showing gas bubbles; b) Close view, showing termites in Colombian copal showing gas bubbles. “Among the most unusual substances on Earth, amber combines alluring physical properties with an unparalleled archive of ancient life on our planet. No other substance brings together such richness in art and science.” David A. Grimaldi, American Museum of Natural History (Ross, 2010) 16 FUNGI Volume 11:5 2019 Figure 2. A rough piece of blue Dominican amber. y quest for mushrooms in Mushroom lovers were treated to a and so versatile. As a gemologist, amber amber and copal lasted about small, extremely rare piece of light- was an organic, semi-precious gemstone a decade, from the mid-1990s colored amber, inside which a tiny gilled for me, soft for a gemstone (only 2-2.5 Mto the early part of the 2000s. During mushroom was barely visible. Through on the Mohs scale), and with multiple this period of time, I found mushrooms a strategically placed magnifying glass, places of origin around the world. It in Mexican red amber from Chiapas, excited visitors could get a glimpse of came in multiple colors, and lended itself and in Colombian copal from the what the ancestor of today’s Mycena to carving and the creation of beautiful Santander region; these pieces make up looked like, down to its cap, delicate works of art. As a mycologist seeking the bulk of my collection. The unique gills, and stipe. Though an extinct mushrooms inside the amber, amber fossils of mushrooms, amber, and copal species and millions of years old, the was the fully polymerized tree resin that in this collection are still waiting to be fossilized mushroom looked fresh and formed millions of years ago in what studied. -

1018Ai Ele788 (18) 12W Rgbwai Led
1018AI ELE788 (18) 12W RGBWAI LED GENERAL INFORMATION A powerful, well-rounded stage light, the elektraLite 1018AI is ready to bring rich vibrant color at a professional level. At the heart of the elektraLite 1018AI are eighteen powerful 12-watt 6-in-1 LED’s, consisting of a red, green, blue, white, amber, and indigo component. The 6-in-1 LED uses diffraction lensing, eliminating “rainbow” color spots which are typical when individual LED’s are exposed in lighting a cyc or similar background. The elektraLite 1018AI is designed to deliver rich, vibrant colors you expect from an LED fixture, while still providing d true, brilliant, tunable white. By mixing color internally, the 1018AI’s sophisticated diffraction lensing system provide true, blended, single-color output. Because of this, the white is tunable with the amber and white LED components, and the color range for pastel and deep blues and purples is intensified with the indigo component. By adding amber and indigo to the LED emitter, the elektraLite 1018AI is ideal for a wide variety of public spaces FEATURES including stages, classrooms, galleries, worship spaces, studios, and event spaces. - LED technology; utilizing eighteen 12-watt 6-IN-1 LED’s - rich, vivid red, blue, green and white, with added amber for A user-friendly onboard control interface allows for multiple true white color toning, and indigo for pastel blending and control options including master/slave, DMX, including RGB, well-rounded color saturation strobe, and console-free static operation. - crisp 5,600K white light and balancing from 2,700K utilizing The 1018AI has 5-pin XLR DMX connectors (both in and amber thru). -

Imitation Amber Beads of Phenolic Resin from the African Trade
IMITATION AMBER BEADS OF PHENOLIC RESIN FROM THE AFRICAN TRADE Rosanna Falabella Examination of contemporary beads with African provenance reveals large quantities of imitation amber beads made of phenol- formaldehyde thermosetting resins (PFs). This article delves into the early industrial history of PFs and their use in the production of imitation amber and bead materials. Attempts to discover actual sources that manufactured imitation amber beads for export to Africa and the time frame have not been very fruitful. While evidence exists that PFs were widely used as amber substitutes within Europe, only a few post-WWII references explicitly report the export of imitation amber PF beads to Africa. However they arrived in Africa, the durability of PF beads gave African beadworkers aesthetic freedom not only to rework the original beads into a variety of shapes and sizes, and impart decorative elements, but also to apply heat treatment to modify colors. Some relatively simple tests to distinguish PFs from other bead materials are presented. Figure 1. Beads of phenol-formaldehyde thermosetting resins INTRODUCTION (PFs) from the African trade. The large bead at bottom center is 52.9 mm in diameter (metric scale) (all images by the author unless otherwise noted). Strands of machined and polished amber-yellow beads, from small to very large (Figure 1), are found today in the He reports that other shapes, such as barrel and spherical stalls of many African bead sellers as well as in on-line (Figure 2), were imported as well, but the short oblates stores and auction sites. They are usually called “African are the most common. -

Uplighting Setup Instructions More Color Options Special Programming
UPLIGHTING SETUP INSTRUCTIONS **Keep all packaging materials. Uplights must be returned in same box / packaging.** SELECT COLOR 1. Use the power cord provided to plug the uplight into a power outlet. 2. Press “MENU” repeatedly until “C” shows on the display. 3. Press “ENTER” 4. Use the “UP” or “DOWN” button to select one of the standard color options: C1 = Red C3 = Blue C5 = Magenta C7 = White C2 = Green C4: =Light Blue C6 = Yellow **For More Colors, See Below 5. Press “ENTER” to save the color. SETUP LIGHTS 1. Place the uplight on the floor, close to a wall or other surface. 2. Tilt light towards wall until desired effect is achieved. Adjust legs by twisting knobs on each side. 3. Link uplights together. Power cords can plug into outlet OR another uplight. Link 40 lights per outlet. MORE COLOR OPTIONS To program a custom color, find your color’s “rgb” code from the chart on the back of this page. Then follow these steps: 1. Press “MENU” repeatedly until “U” shows on the display. Press “ENTER” 2. You should see “r” with a number. Press “UP” or “DOWN” to set the “r” value for your color. Press “ENTER” 3. You should see “g” with a number. Press “UP” or “DOWN” to set your “g” value. Press “ENTER” 4. You should see “b” with a number. Press “UP” or “DOWN” to set your “b” value. Press ENTER. You’re Done! SPECIAL PROGRAMMING Sound Active Mode 1. Press the MENU button repeatedly until “P—“ shows on the display. Press ENTER. 2.