Network Scan Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Predatory Mite (Acari, Parasitiformes: Mesostigmata (Gamasina); Acariformes: Prostigmata) Community in Strawberry Agrocenosis
Acta Universitatis Latviensis, Biology, 2004, Vol. 676, pp. 87–95 The predatory mite (Acari, Parasitiformes: Mesostigmata (Gamasina); Acariformes: Prostigmata) community in strawberry agrocenosis Valentîna Petrova*, Ineta Salmane, Zigrîda Çudare Institute of Biology, University of Latvia, Miera 3, Salaspils LV-2169, Latvia *Corresponding author, E-mail: [email protected]. Abstract Altogether 37 predatory mite species from 14 families (Parasitiformes and Acariformes) were collected using leaf sampling and pit-fall trapping in strawberry fi elds (1997 - 2001). Thirty- six were recorded on strawberries for the fi rst time in Latvia. Two species, Paragarmania mali (Oud.) (Aceosejidae) and Eugamasus crassitarsis (Hal.) (Parasitidae) were new for the fauna of Latvia. The most abundant predatory mite families (species) collected from strawberry leaves were Phytoseiidae (Amblyseius cucumeris Oud., A. aurescens A.-H., A. bicaudus Wainst., A. herbarius Wainst.) and Anystidae (Anystis baccarum L.); from pit-fall traps – Parasitidae (Poecilochirus necrophori Vitz. and Parasitus lunaris Berl.), Aceosejidae (Leioseius semiscissus Berl.) and Macrochelidae (Macrocheles glaber Müll). Key words: agrocenosis, diversity, predatory mites, strawberry. Introduction Predatory mites play an important ecological role in terrestrial ecosystems and they are increasingly being used in management for biocontrol of pest mites, thrips and nematodes (Easterbrook 1992; Wright, Chambers 1994; Croft et al. 1998; Cuthbertson et al. 2003). Many of these mites have a major infl uence on nutrient cycling, as they are predators on other arthropods (Santos 1985; Karg 1993; Koehler 1999). In total, investigations of mite fauna in Latvia were made by Grube (1859), who found 28 species, Eglītis (1954) – 50 species, Kuznetsov and Petrov (1984) – 85 species, Lapiņa (1988) – 207 species, and Salmane (2001) – 247 species. -

NSW Rainforest Trees Part
This document has been scanned from hard-copy archives for research and study purposes. Please note not all information may be current. We have tried, in preparing this copy, to make the content accessible to the widest possible audience but in some cases we recognise that the automatic text recognition maybe inadequate and we apologise in advance for any inconvenience this may cause. · RESEARCH NOTE No. 35 ~.I~=1 FORESTRY COMMISSION OF N.S.W. RESEARCH NOTE No. 35 P)JBLISHED 197R N.S.W. RAINFOREST TREES PART VII FAMILIES: PROTEACEAE SANTALACEAE NYCTAGINACEAE GYROSTEMONACEAE ANNONACEAE EUPOMATIACEAE MONIMIACEAE AUTHOR A.G.FLOYD (Research Note No. 35) National Library of Australia card number and ISBN ISBN 0 7240 13997 ISSN 0085-3984 INTRODUCTION This is the seventh in a series ofresearch notes describing the rainforest trees of N.S. W. Previous publications are:- Research Note No. 3 (I 960)-N.S.W. Rainforest Trees. Part I Family LAURACEAE. A. G. Floyd and H. C. Hayes. Research Note No. 7 (1961)-N.S.W. Rainforest Trees. Part II Families Capparidaceae, Escalloniaceae, Pittosporaceae, Cunoniaceae, Davidsoniaceae. A. G. Floyd and H. C. Hayes. Research Note No. 28 (I 973)-N.S.W. Rainforest Trees. Part III Family Myrtaceae. A. G. Floyd. Research Note No. 29 (I 976)-N.S.W. Rainforest Trees. Part IV Family Rutaceae. A. G. Floyd. Research Note No. 32 (I977)-N.S.W. Rainforest Trees. Part V Families Sapindaceae, Akaniaceae. A. G. Floyd. Research Note No. 34 (1977)-N.S.W. Rainforest Trees. Part VI Families Podocarpaceae, Araucariaceae, Cupressaceae, Fagaceae, Ulmaceae, Moraceae, Urticaceae. -

Insecticides - Development of Safer and More Effective Technologies
INSECTICIDES - DEVELOPMENT OF SAFER AND MORE EFFECTIVE TECHNOLOGIES Edited by Stanislav Trdan Insecticides - Development of Safer and More Effective Technologies http://dx.doi.org/10.5772/3356 Edited by Stanislav Trdan Contributors Mahdi Banaee, Philip Koehler, Alexa Alexander, Francisco Sánchez-Bayo, Juliana Cristina Dos Santos, Ronald Zanetti Bonetti Filho, Denilson Ferrreira De Oliveira, Giovanna Gajo, Dejane Santos Alves, Stuart Reitz, Yulin Gao, Zhongren Lei, Christopher Fettig, Donald Grosman, A. Steven Munson, Nabil El-Wakeil, Nawal Gaafar, Ahmed Ahmed Sallam, Christa Volkmar, Elias Papadopoulos, Mauro Prato, Giuliana Giribaldi, Manuela Polimeni, Žiga Laznik, Stanislav Trdan, Shehata E. M. Shalaby, Gehan Abdou, Andreia Almeida, Francisco Amaral Villela, João Carlos Nunes, Geri Eduardo Meneghello, Adilson Jauer, Moacir Rossi Forim, Bruno Perlatti, Patrícia Luísa Bergo, Maria Fátima Da Silva, João Fernandes, Christian Nansen, Solange Maria De França, Mariana Breda, César Badji, José Vargas Oliveira, Gleberson Guillen Piccinin, Alan Augusto Donel, Alessandro Braccini, Gabriel Loli Bazo, Keila Regina Hossa Regina Hossa, Fernanda Brunetta Godinho Brunetta Godinho, Lilian Gomes De Moraes Dan, Maria Lourdes Aldana Madrid, Maria Isabel Silveira, Fabiola-Gabriela Zuno-Floriano, Guillermo Rodríguez-Olibarría, Patrick Kareru, Zachaeus Kipkorir Rotich, Esther Wamaitha Maina, Taema Imo Published by InTech Janeza Trdine 9, 51000 Rijeka, Croatia Copyright © 2013 InTech All chapters are Open Access distributed under the Creative Commons Attribution 3.0 license, which allows users to download, copy and build upon published articles even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. After this work has been published by InTech, authors have the right to republish it, in whole or part, in any publication of which they are the author, and to make other personal use of the work. -
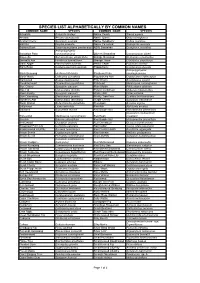
Species List Alphabetically by Common Names
SPECIES LIST ALPHABETICALLY BY COMMON NAMES COMMON NAME SPECIES COMMON NAME SPECIES Actephila Actephila lindleyi Native Peach Trema aspera Ancana Ancana stenopetala Native Quince Guioa semiglauca Austral Cherry Syzygium australe Native Raspberry Rubus rosifolius Ball Nut Floydia praealta Native Tamarind Diploglottis australis Banana Bush Tabernaemontana pandacaqui NSW Sassafras Doryphora sassafras Archontophoenix Bangalow Palm cunninghamiana Oliver's Sassafras Cinnamomum oliveri Bauerella Sarcomelicope simplicifolia Orange Boxwood Denhamia celastroides Bennetts Ash Flindersia bennettiana Orange Thorn Citriobatus pauciflorus Black Apple Planchonella australis Pencil Cedar Polyscias murrayi Black Bean Castanospermum australe Pepperberry Cryptocarya obovata Archontophoenix Black Booyong Heritiera trifoliolata Picabeen Palm cunninghamiana Black Wattle Callicoma serratifolia Pigeonberry Ash Cryptocarya erythroxylon Blackwood Acacia melanoxylon Pink Cherry Austrobuxus swainii Bleeding Heart Omalanthus populifolius Pinkheart Medicosma cunninghamii Blue Cherry Syzygium oleosum Plum Myrtle Pilidiostigma glabrum Blue Fig Elaeocarpus grandis Poison Corkwood Duboisia myoporoides Blue Lillypilly Syzygium oleosum Prickly Ash Orites excelsa Blue Quandong Elaeocarpus grandis Prickly Tree Fern Cyathea leichhardtiana Blueberry Ash Elaeocarpus reticulatus Purple Cherry Syzygium crebrinerve Blush Walnut Beilschmiedia obtusifolia Red Apple Acmena ingens Bollywood Litsea reticulata Red Ash Alphitonia excelsa Bolwarra Eupomatia laurina Red Bauple Nut Hicksbeachia -

Proceedings of a Workshop on Biodiversity Dynamics on La Réunion Island
PROCEEDINGS OF A WORKSHOP ON BIODIVERSITY DYNAMICS ON LA RÉUNION ISLAND ATELIER SUR LA DYNAMIQUE DE LA BIODIVERSITE A LA REUNION SAINT PIERRE – SAINT DENIS 29 NOVEMBER – 5 DECEMBER 2004 29 NOVEMBRE – 5 DECEMBRE 2004 T. Le Bourgeois Editors Stéphane Baret, CIRAD UMR C53 PVBMT, Réunion, France Mathieu Rouget, National Biodiversity Institute, South Africa Ingrid Nänni, National Biodiversity Institute, South Africa Thomas Le Bourgeois, CIRAD UMR C53 PVBMT, Réunion, France Workshop on Biodiversity dynamics on La Reunion Island - 29th Nov. to 5th Dec. 2004 WORKSHOP ON BIODIVERSITY DYNAMICS major issues: Genetics of cultivated plant ON LA RÉUNION ISLAND species, phytopathology, entomology and ecology. The research officer, Monique Rivier, at Potential for research and facilities are quite French Embassy in Pretoria, after visiting large. Training in biology attracts many La Réunion proposed to fund and support a students (50-100) in BSc at the University workshop on Biodiversity issues to develop (Sciences Faculty: 100 lecturers, 20 collaborations between La Réunion and Professors, 2,000 students). Funding for South African researchers. To initiate the graduate grants are available at a regional process, we decided to organise a first or national level. meeting in La Réunion, regrouping researchers from each country. The meeting Recent cooperation agreements (for was coordinated by Prof D. Strasberg and economy, research) have been signed Dr S. Baret (UMR CIRAD/La Réunion directly between La Réunion and South- University, France) and by Prof D. Africa, and former agreements exist with Richardson (from the Institute of Plant the surrounding Indian Ocean countries Conservation, Cape Town University, (Madagascar, Mauritius, Comoros, and South Africa) and Dr M. -

A NEW SPECIES of LORRYIA (ACARI: TYDEIDAE) from a TERMITE NEST in SOUTH AFRICA Alexander A
Acarina 28 (1): 47–53 © Acarina 2020 A NEW SPECIES OF LORRYIA (ACARI: TYDEIDAE) FROM A TERMITE NEST IN SOUTH AFRICA Alexander A. Khaustov1*, Elizabeth A. Hugo-Coetzee2, 3 and Sergey G. Ermilov1 1X-BIO Institute, Tyumen State University, Tyumen, Russia 2Terrestrial Invertebrate Department, National Museum, Bloemfontein, South Africa 3Department of Zoology and Entomology, University of the Free State, Bloemfontein, South Africa *corresponding author; e–mail: [email protected] ABSTRACT: A new species—Lorryia pseudoplacita sp. n.—is described from central South Africa. It was collected from a nest of the termite Trinervitermes trinervoides (Sjöstedt, 1911) (Isoptera: Termitidae). The new species differs from L. placita in the shape of the dorsal setae and in having a shorter palptarsus. KEY WORDS: Tydeoidea, systematics, morphology, SEM microscopy, Ethiopian region. DOI: 10.21684/0132-8077-2020-28-1-47-53 INTRODUCTION The family Tydeidae currently includes more Khaustov et al. 2017a, b, 2018a, b, 2019a, b). Du- than 300 species, sorted into about 30 genera; it is ring the study of mites inhabiting termite nests in a diverse group of mostly fungivorous mites, char- South Africa, a new species of Lorryia was discov- acterized by a worldwide distribution (Silva et al. ered in the nest of Trinervitermes trinervoides 2016; Kaźmierski et al. 2018). Twenty-one species (Sjöstedt, 1911) (Isoptera: Termitidae). The aim of of Tydeidae have been described from South Af- this paper is to describe the new species and to rica: Pretydeus curiosa (Ueckermann and Smith compare it with the holotype of the closely related Meyer, 1979), Ueckermannia grewiae (Uecker- L. placita (Livshitz, 1973). -

2017 Summit + SES Meeting
2017 Summit + SES Meeting June 5th - 9th @ Colorado State University Fort Collins, Colorado elcome to the Ecology of Soil Health Summit and the biennial meeting of the Soil Ecology Society. This meeting comes at a time when interest Win soil health–a concept that embraces not only the importance of soil organic matter content and physical structure, but also the key role of soil biology for productive and efficient agroecosystems–is rapidly growing. In parallel, our scientific understanding of the ecology of the soil system has advanced tremendously in recent years, in many cases challenging longstanding paradigms. This international summit provides a unique opportunity to advance the development and implementation of science- based programs that enhance Soil Health. I hope that all meeting participants come away with a broader perspective, new ideas and new collaborators. It has been my pleasure to help organize this meeting, and I thank you for joining us. Matthew Wallenstein, PhD President, Soil Ecology Society Director, Innovation Center for Sustainable Agriculture Acknowledgements Chairman Matthew Wallenstein Program Assistant Laurie Richards Soil Ecology Society Conference Committee Loren Byrne Samantha Chapman Jen Krumins Michael Weintraub Ecology of Soil Health Summit Committee Nicholas Goeser Mary Beth Miranda Whendee Silver Allison Thomson Local Conference Committee Charlotte Alster Courtland Kelly Yamina Pressler Summit Supporters Summit Advocates The Soil Ecology Society (SES) is an international professional organization dedicated to furthering the science, education and awareness of soil ecology and the importance of soils for human and environmental well-being. Commitment to Diversity Just as healthy soils rely on interactive networks of incredibly diverse organisms, SES depends on all dimensions of human diversity. -

Niche Modeling May Explain the Historical Population Failure of Phytoseiulus Persimilis in Taiwan: Implications of Biocontrol Strategies
insects Article Niche Modeling May Explain the Historical Population Failure of Phytoseiulus persimilis in Taiwan: Implications of Biocontrol Strategies Jhih-Rong Liao 1 , Chyi-Chen Ho 2, Ming-Chih Chiu 3,* and Chiung-Cheng Ko 1,† 1 Department of Entomology, National Taiwan University, Taipei 106332, Taiwan; [email protected] (J.-R.L.); [email protected] (C.-C.K.) 2 Taiwan Acari Research Laboratory, Taichung 413006, Taiwan; [email protected] 3 Center for Marine Environmental Studies (CMES), Ehime University, Matsuyama 7908577, Japan * Correspondence: [email protected] † Deceased, 29 October 2020. This paper is dedicated to the memory of the late Chiung-Cheng Ko. Simple Summary: Phytoseiulus persimilis Athias-Henriot, a mite species widely used in pest manage- ment for the control of spider mites, has been commercialized and introduced to numerous countries. In the 1990s, P. persimilis was imported to Taiwan, and a million individuals were released into the field. However, none have been observed since then. In this study, we explored the ecological niche of this species to determine reasons underlying its establishment failure. The results indicate that P. persimilis was released in areas poorly suited to their survival. To the best of our knowledge, the present study is the first to predict the potential distribution of phytoseiids as exotic natural enemies. This process should precede the commercialization of exotic natural enemies and their introduction Citation: Liao, J.-R.; Ho, C.-C.; Chiu, into any country. M.-C.; Ko, C.-C. Niche Modeling May Explain the Historical Population Abstract: Biological control commonly involves the commercialization and introduction of natural Failure of Phytoseiulus persimilis in enemies. -

Volume: 1 Issue: 2 Year: 2019
Volume: 1 Issue: 2 Year: 2019 Designed by Müjdat TÖS Acarological Studies Vol 1 (2) CONTENTS Editorial Acarological Studies: A new forum for the publication of acarological works ................................................................... 51-52 Salih DOĞAN Review An overview of the XV International Congress of Acarology (XV ICA 2018) ........................................................................ 53-58 Sebahat K. OZMAN-SULLIVAN, Gregory T. SULLIVAN Articles Alternative control agents of the dried fruit mite, Carpoglyphus lactis (L.) (Acari: Carpoglyphidae) on dried apricots ......................................................................................................................................................................................................................... 59-64 Vefa TURGU, Nabi Alper KUMRAL A species being worthy of its name: Intraspecific variations on the gnathosomal characters in topotypic heter- omorphic males of Cheylostigmaeus variatus (Acari: Stigmaeidae) ........................................................................................ 65-70 Salih DOĞAN, Sibel DOĞAN, Qing-Hai FAN Seasonal distribution and damage potential of Raoiella indica (Hirst) (Acari: Tenuipalpidae) on areca palms of Kerala, India ............................................................................................................................................................................................................... 71-83 Prabheena PRABHAKARAN, Ramani NERAVATHU Feeding impact of Cisaberoptus -

A Snap-Shot of Domatial Mite Diversity of Coffea Arabica in Comparison to the Adjacent Umtamvuna Forest in South Africa
diversity Article A Snap-Shot of Domatial Mite Diversity of Coffea arabica in Comparison to the Adjacent Umtamvuna Forest in South Africa 1, , 2 1 Sivuyisiwe Situngu * y, Nigel P. Barker and Susanne Vetter 1 Botany Department, Rhodes University, P.O. Box 94, Makhanda 6139, South Africa; [email protected] 2 Department of Plant and Soil Sciences, University of Pretoria, P. Bag X20, Hatfield 0028, South Africa; [email protected] * Correspondence: [email protected]; Tel.: +27-(0)11-767-6340 Present address: School of Animal, Plant and Environmental Sciences, University of Witwatersrand, y Private Bag 3, Johannesburg 2050, South Africa. Received: 21 January 2020; Accepted: 14 February 2020; Published: 18 February 2020 Abstract: Some plant species possess structures known as leaf domatia, which house mites. The association between domatia-bearing plants and mites has been proposed to be mutualistic, and has been found to be important in species of economic value, such as grapes, cotton, avocado and coffee. This is because leaf domatia affect the distribution, diversity and abundance of predatory and mycophagous mites found on the leaf surface. As a result, plants are thought to benefit from increased defence against pathogens and small arthropod herbivores. This study assesses the relative diversity and composition of mites on an economically important plant host (Coffea aribica) in comparison to mites found in a neighbouring indigenous forest in South Africa. Our results showed that the coffee plantations were associated with only predatory mites, some of which are indigenous to South Africa. This indicates that coffee plantations are able to be successfully colonised by indigenous beneficial mites. -

Red Palm Mite)
Crop Protection Compendium Datasheet report for Raoiella indica (red palm mite) Top of page Pictures Picture Title Caption Copyright Adult The red palm mite (Raoiella indica), an invasive species in the Caribbean, may threaten USDA- mite several important palms found in the southern USA. (Original magnified approx. 300x.) ARS Photo by Eric Erbe; Digital colourization by Chris Pooley. Colony Colony of red palm mites (Raoiella indica) on coconut leaflet, from India. Bryony of Taylor mites Colony Close-up of a colony of red palm mites (Raoiella indica) on coconut leaflet, from India. Bryony of Taylor mites Top of page Identity Preferred Scientific Name Raoiella indica Hirst (1924) Preferred Common Name red palm mite International Common Names English: coconut red mite; frond crimson mite; leaflet false spider mite; red date palm mite; scarlet mite EPPO code RAOIIN (Raoiella indica) Top of page Taxonomic Tree Domain: Eukaryota Kingdom: Metazoa Phylum: Arthropoda Subphylum: Chelicerata Class: Arachnida Subclass: Acari Superorder: Acariformes Suborder: Prostigmata Family: Tenuipalpidae Genus: Raoiella Species: Raoiella indica / Top of page Notes on Taxonomy and Nomenclature R. indica was first described in the district of Coimbatore (India) by Hirst in 1924 on coconut leaflets [Cocos nucifera]. A comprehensive taxonomic review of the genus and species was carried out by Mesa et al. (2009), which lists all suspected junior synonyms of R. indica, including Raoiella camur (Chaudhri and Akbar), Raoiella empedos (Chaudhri and Akbar), Raoiella obelias (Hasan and Akbar), Raoiella pandanae (Mohanasundaram), Raoiella phoenica (Meyer) and Raoiella rahii (Akbar and Chaudhri). The review also highlighted synonymy with Rarosiella cocosae found on coconut in the Philippines. -

Unexpected Effects of Local Management and Landscape Composition on Predatory Mites and Their Food Resources in Vineyards
insects Article Unexpected Effects of Local Management and Landscape Composition on Predatory Mites and Their Food Resources in Vineyards Stefan Möth 1,* , Andreas Walzer 1, Markus Redl 1, Božana Petrovi´c 1, Christoph Hoffmann 2 and Silvia Winter 1 1 Institute of Plant Protection, University of Natural Resources and Life Sciences Vienna (BOKU), Gregor-Mendel-Straße 33, 1180 Vienna, Austria; [email protected] (A.W.); [email protected] (M.R.); [email protected] (B.P.); [email protected] (S.W.) 2 Julius Kühn-Institute (JKI), Institute for Plant Protection in Fruit Crops and Viticulture, Geilweilerhof, 76833 Siebeldingen, Germany; [email protected] * Correspondence: [email protected]; Tel.: +43-1-47654-95329 Simple Summary: Sustainable agriculture becomes more important for biodiversity conservation and environmental protection. Viticulture is characterized by relatively high pesticide inputs, which could decrease arthropod populations and biological pest control in vineyards. This problem could be counteracted with management practices such as the implementation of diverse vegetation cover in the vineyard inter-rows, reduced pesticide input in integrated or organic vineyards, and a di- verse landscape with trees and hedges. We examined the influence of these factors on predatory Citation: Möth, S.; Walzer, A.; Redl, mites, which play a crucial role as natural enemies for pest mites on vines, and pollen as impor- M.; Petrovi´c,B.; Hoffmann, C.; Winter, tant alternative food source for predatory mites in 32 organic and integrated Austrian vineyards. S. Unexpected Effects of Local Predatory mites benefited from integrated pesticide management and spontaneous vegetation cover Management and Landscape in vineyard inter-rows.