Flowering Plants. Eudicots
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Well-Known Plants in Each Angiosperm Order
Well-known plants in each angiosperm order This list is generally from least evolved (most ancient) to most evolved (most modern). (I’m not sure if this applies for Eudicots; I’m listing them in the same order as APG II.) The first few plants are mostly primitive pond and aquarium plants. Next is Illicium (anise tree) from Austrobaileyales, then the magnoliids (Canellales thru Piperales), then monocots (Acorales through Zingiberales), and finally eudicots (Buxales through Dipsacales). The plants before the eudicots in this list are considered basal angiosperms. This list focuses only on angiosperms and does not look at earlier plants such as mosses, ferns, and conifers. Basal angiosperms – mostly aquatic plants Unplaced in order, placed in Amborellaceae family • Amborella trichopoda – one of the most ancient flowering plants Unplaced in order, placed in Nymphaeaceae family • Water lily • Cabomba (fanwort) • Brasenia (watershield) Ceratophyllales • Hornwort Austrobaileyales • Illicium (anise tree, star anise) Basal angiosperms - magnoliids Canellales • Drimys (winter's bark) • Tasmanian pepper Laurales • Bay laurel • Cinnamon • Avocado • Sassafras • Camphor tree • Calycanthus (sweetshrub, spicebush) • Lindera (spicebush, Benjamin bush) Magnoliales • Custard-apple • Pawpaw • guanábana (soursop) • Sugar-apple or sweetsop • Cherimoya • Magnolia • Tuliptree • Michelia • Nutmeg • Clove Piperales • Black pepper • Kava • Lizard’s tail • Aristolochia (birthwort, pipevine, Dutchman's pipe) • Asarum (wild ginger) Basal angiosperms - monocots Acorales -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

A Visual Guide to Collecting Plant Tissues for DNA
A visual guide to collecting plant tissues for DNA Collecting kit checklist Silica gel1 Permanent marker and pencil Resealable bags, airtight plastic container Razor blade / Surgical scissors Empty tea bags or coffee filters Ethanol and paper tissue or ethanol wipes Tags or jewellers tags Plant press and collecting book 1. Selection and preparation of fresh plant tissue: Sampling avoided. Breaking up leaf material will bruise the plant tissue, which will result in enzymes being released From a single plant, harvest 3 – 5 mature leaves, or that cause DNA degradation. Ideally, leaf material sample a piece of a leaf, if large (Picture A). Ideally should be cut into smaller fragments with thick a leaf area of 5 – 10 cm2 should be enough, but this midribs being removed (Picture C). If sampling robust amount should be adjusted if the plant material is leaf tissue (e.g. cycads, palms), use a razor blade or rich in water (e.g. a succulent plant). If leaves are surgical scissors (Picture D). small (e.g. ericoid leaves), sample enough material to equate a leaf area of 5 – 10 cm2. If no leaves are Succulent plants available, other parts can be sampled such as leaf buds, flowers, bracts, seeds or even fresh bark. If the If the leaves are succulent, use a razor blade to plant is small, select the biggest specimen, but never remove epidermal slices or scoop out parenchyma combine tissues from different individuals. tissue (Picture E). Cleaning Ideally, collect clean fresh tissues, however if the leaf or plant material is dirty or shows potential contamination (e.g. -

GENOME EVOLUTION in MONOCOTS a Dissertation
GENOME EVOLUTION IN MONOCOTS A Dissertation Presented to The Faculty of the Graduate School At the University of Missouri In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy By Kate L. Hertweck Dr. J. Chris Pires, Dissertation Advisor JULY 2011 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled GENOME EVOLUTION IN MONOCOTS Presented by Kate L. Hertweck A candidate for the degree of Doctor of Philosophy And hereby certify that, in their opinion, it is worthy of acceptance. Dr. J. Chris Pires Dr. Lori Eggert Dr. Candace Galen Dr. Rose‐Marie Muzika ACKNOWLEDGEMENTS I am indebted to many people for their assistance during the course of my graduate education. I would not have derived such a keen understanding of the learning process without the tutelage of Dr. Sandi Abell. Members of the Pires lab provided prolific support in improving lab techniques, computational analysis, greenhouse maintenance, and writing support. Team Monocot, including Dr. Mike Kinney, Dr. Roxi Steele, and Erica Wheeler were particularly helpful, but other lab members working on Brassicaceae (Dr. Zhiyong Xiong, Dr. Maqsood Rehman, Pat Edger, Tatiana Arias, Dustin Mayfield) all provided vital support as well. I am also grateful for the support of a high school student, Cady Anderson, and an undergraduate, Tori Docktor, for their assistance in laboratory procedures. Many people, scientist and otherwise, helped with field collections: Dr. Travis Columbus, Hester Bell, Doug and Judy McGoon, Julie Ketner, Katy Klymus, and William Alexander. Many thanks to Barb Sonderman for taking care of my greenhouse collection of many odd plants brought back from the field. -

Phylogeny of Rosids! ! Rosids! !
Phylogeny of Rosids! Rosids! ! ! ! ! Eurosids I Eurosids II Vitaceae Saxifragales Eurosids I:! Eurosids II:! Zygophyllales! Brassicales! Celastrales! Malvales! Malpighiales! Sapindales! Oxalidales! Myrtales! Fabales! Geraniales! Rosales! Cucurbitales! Fagales! After Jansen et al., 2007, Proc. Natl. Acad. Sci. USA 104: 19369-19374! Phylogeny of Rosids! Rosids! ! ! ! ! Eurosids I Eurosids II Vitaceae Saxifragales Eurosids I:! Eurosids II:! Zygophyllales! Brassicales! Celastrales! Malvales! Malpighiales! Sapindales! Oxalidales! Myrtales! Fabales! Geraniales! Rosales! Cucurbitales! Fagales! After Jansen et al., 2007, Proc. Natl. Acad. Sci. USA 104: 19369-19374! Alnus - alders A. rubra A. rhombifolia A. incana ssp. tenuifolia Alnus - alders Nitrogen fixation - symbiotic with the nitrogen fixing bacteria Frankia Alnus rubra - red alder Alnus rhombifolia - white alder Alnus incana ssp. tenuifolia - thinleaf alder Corylus cornuta - beaked hazel Carpinus caroliniana - American hornbeam Ostrya virginiana - eastern hophornbeam Phylogeny of Rosids! Rosids! ! ! ! ! Eurosids I Eurosids II Vitaceae Saxifragales Eurosids I:! Eurosids II:! Zygophyllales! Brassicales! Celastrales! Malvales! Malpighiales! Sapindales! Oxalidales! Myrtales! Fabales! Geraniales! Rosales! Cucurbitales! Fagales! After Jansen et al., 2007, Proc. Natl. Acad. Sci. USA 104: 19369-19374! Fagaceae (Beech or Oak family) ! Fagaceae - 9 genera/900 species.! Trees or shrubs, mostly northern hemisphere, temperate region ! Leaves simple, alternate; often lobed, entire or serrate, deciduous -

583–584 Angiosperms 583 *Eudicots and Ceratophyllales
583 583 > 583–584 Angiosperms These schedules are extensively revised, having been prepared with little reference to earlier editions. 583 *Eudicots and Ceratophyllales Subdivisions are added for eudicots and Ceratophyllales together, for eudicots alone Class here angiosperms (flowering plants), core eudicots For monocots, basal angiosperms, Chloranthales, magnoliids, see 584 See Manual at 583–585 vs. 600; also at 583–584; also at 583 vs. 582.13 .176 98 Mangrove swamp ecology Number built according to instructions under 583–588 Class here comprehensive works on mangroves For mangroves of a specific order or family, see the order or family, e.g., mangroves of family Combretaceae 583.73 .2 *Ceratophyllales Class here Ceratophyllaceae Class here hornworts > 583.3–583.9 Eudicots Class comprehensive works in 583 .3 *Ranunculales, Sabiaceae, Proteales, Trochodendrales, Buxales .34 *Ranunculales Including Berberidaceae, Eupteleaceae, Menispermaceae, Ranunculaceae Including aconites, anemones, barberries, buttercups, Christmas roses, clematises, columbines, delphiniums, hellebores, larkspurs, lesser celandine, mandrake, mayapple, mayflower, monkshoods, moonseeds, wolfsbanes For Fumariaceae, Papaveraceae, Pteridophyllaceae, see 583.35 See also 583.9593 for mandrakes of family Solanaceae .35 *Fumariaceae, Papaveraceae, Pteridophyllaceae Including bleeding hearts, bloodroot, celandines, Dutchman’s breeches, fumitories, poppies See also 583.34 for lesser celandine .37 *Sabiaceae * *Add as instructed under 583–588 1 583 Dewey Decimal Classification -
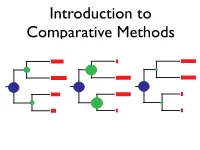
BM CC EB What Can We Learn from a Tree?
Introduction to Comparative Methods BM CC EB What can we learn from a tree? Net diversification (r) Relative extinction (ε) Peridiscaceae Peridiscaceae yllaceae yllaceae h h atop atop Proteaceae Proteaceae r r Ce Ce Tr oc T ho r M M o de c y y H H C C h r r e e a D D a o o nd o e e G G a a m m t t a a d r P r P h h e e u u c c p A p A r e a a e e a a a c a c n n a i B i B h h n d m d l m a l a m m e a e a e e t t n n c u u n n i i d i i e e e e o n o n p n p n a e a S e e S e e n n x x i i r c c a a n o n o p p h g e h g ae e l r a l r a a a a a a i i a a a e a e i b i b h y d c h d c i y i c a a c x c x c c G I a G I a n c n c c c y l y l t a a t a a e e e e e i l c i l c m l m l e c e c f a e a a f a e a a l r r l c c a i i r l e t e t a a r l a a e e u u u u o a o a a a c a c a a l a l e e e b b a a a a e e c e e c a a s c s c c e l c e l e e g e g e a a a a e e n n s e e s e e e e a a a P a P e e N N u u u S u S a e a e a a e e c c l n a l n e e a e e a e a e a e a e r a r a c c C i C i R R a e a e a e a e r c r c A A a d a a d a e i e i phanopetalaceae s r e ph s r e a a s e c s e c e e u u b a a b a e e P P r r l l e n e a a a a m m entho e e e Ha a H o a c r e c r e nt B B e p e e e c e e c a c c h e a p a a p a lo lo l l a a e s o t e s i a r a i a r r r r r a n e a n e a b a l b t a t gaceae e g e ceae a c a s c s a z e z M i a e M i a c a d e a d e ae e ae r e r a e a e a a a c c ce r e r L L i i ac a Vitaceae Vi r r C C e e ta v e v e a a c a a e ea p e ap c a c a e a P P e e l l e Ge G e e ae a t t e e p p r r ce c an u an -

Supplementary Information
Supporting Information for Cornwell et al. 2014. Functional distinctiveness of major 1 plant lineages. doi: 10.1111/1365-2745.12208. SUPPORTING INFORMATION Summary In this Supporting Information, we report additional methods details with respect to the trait data, the climate data, and the analysis. In addition, to more fully explain the method, we include several additional figures. We show the top-five lineages, including the population of lineages from which they were selected, for five important traits (Figure S1 on five separate pages); the bivariate distribution of three selected clades with respect to SLA and leaf N, components of the leaf economic spectrum (Figure S2), the geographic distribution of the clades where this proved useful for interpretation (Figure S3); the procedure for selecting the top six nodes in the leaf N trait, to illustrate the internal behavior of our new comparative method, especially the relative contribution of components of extremeness and the sample size weighting to the results (Figure S4). Finally, we provide references for data used in analyses. SUPPORTING METHODS TRAITS DATABASE We compiled a database for five plant functional traits. Each of these traits is the result of a separate research initiative in which data were gathered directly from researchers leading those efforts and/or the literature; in most, but not all cases, these data have been published elsewhere. Detailed methods for data collection and assembly for each trait are available in the original publications; further data were added for some traits from the primary literature (for references see description of individual traits and Supporting References below). For our compilation, all data were brought to common units for a given trait and thoroughly error checked. -

Wildflowers and Ferns Along the Acton Arboretum Wildflower Trail and in Other Gardens FERNS (Including Those Occurring Naturally
Wildflowers and Ferns Along the Acton Arboretum Wildflower Trail and In Other Gardens Updated to June 9, 2018 by Bruce Carley FERNS (including those occurring naturally along the trail and both boardwalks) Royal fern (Osmunda regalis): occasional along south boardwalk, at edge of hosta garden, and elsewhere at Arboretum Cinnamon fern (Osmunda cinnamomea): naturally occurring in quantity along south boardwalk Interrupted fern (Osmunda claytoniana): naturally occurring in quantity along south boardwalk Maidenhair fern (Adiantum pedatum): several healthy clumps along boardwalk and trail, a few in other Arboretum gardens Common polypody (Polypodium virginianum): 1 small clump near north boardwalk Hayscented fern (Dennstaedtia punctilobula): aggressive species; naturally occurring along north boardwalk Bracken fern (Pteridium aquilinum): occasional along wildflower trail; common elsewhere at Arboretum Broad beech fern (Phegopteris hexagonoptera): up to a few near north boardwalk; also in rhododendron and hosta gardens New York fern (Thelypteris noveboracensis): naturally occurring and abundant along wildflower trail * Ostrich fern (Matteuccia pensylvanica): well-established along many parts of wildflower trail; fiddleheads edible Sensitive fern (Onoclea sensibilis): naturally occurring and abundant along south boardwalk Lady fern (Athyrium filix-foemina): moderately present along wildflower trail and south boardwalk Common woodfern (Dryopteris spinulosa): 1 patch of 4 plants along south boardwalk; occasional elsewhere at Arboretum Marginal -

Sabiales Nymphaeales Austrobaileyales
Amborellales Sabiales Nymphaeales Austrobaileyales Acorales G Eenzaadlobbigen G Alismatales Petrosaviales De hier behandelde ordes vorm Pandanales lobbigen. Ze zijn samen geen ec Dioscoreales vroege ontwikkeling van de tw Liliales Asparagales Sabiales: De orde van de Sabial Arecales werd gerekend. Zijn plek in de Dasypogonales Poales Verschillende genanalyses geve G Commeliniden G Commelinales buurt van de andere ordes die h Zingiberales Ceratophyllales Proteales: Deze orde heeft een (Elaeagnaceae) is naar de Rosal Chloranthales enige overgebleven familie, de Canellales Plataanfamilie (Platanaceae). De Piperales G Magnoliiden G Deze orde wordt ondersteund d Magnoliales Laurales meer 2-tallige bloemen hebben Ranunculales Sabiales Sabiaceae Proteales Trochodendrales Nelumbonaceae Buxales Proteaceae Platanaceae Gunnerales Berberidopsidales Trochodendraceae Dilleniales Caryophyllales Buxaceae Santalales Saxifragales G Geavanceerde tweezaadlobbigen G Vitales (Euphorbiaceae) en enkele ande Crossosomatales kenmerken van de bloemen en Geraniales dus in de Rosiden. De Buxales z Myrtales Trochodendrales geplaatst. Zygophyllales Celastrales Malpighiales G G Fabiden Oxalidales The orders treated here are called ‘p Fabales natural group, but represent a series Rosales Cucurbitales Fagales Sabiales: The order Sabiales contain Its position in the dicots is not yet q Brassicales G G but all place Sabiales near the other Malviden Malvales Sapindales Proteales: This order has a complete and two surprising newcomers have Cornales Nelumbonaceae and Platanaceae. -

Tracheophyte of Xiao Hinggan Ling in China: an Updated Checklist
Biodiversity Data Journal 7: e32306 doi: 10.3897/BDJ.7.e32306 Taxonomic Paper Tracheophyte of Xiao Hinggan Ling in China: an updated checklist Hongfeng Wang‡§, Xueyun Dong , Yi Liu|,¶, Keping Ma | ‡ School of Forestry, Northeast Forestry University, Harbin, China § School of Food Engineering Harbin University, Harbin, China | State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences, Beijing, China ¶ University of Chinese Academy of Sciences, Beijing, China Corresponding author: Hongfeng Wang ([email protected]) Academic editor: Daniele Cicuzza Received: 10 Dec 2018 | Accepted: 03 Mar 2019 | Published: 27 Mar 2019 Citation: Wang H, Dong X, Liu Y, Ma K (2019) Tracheophyte of Xiao Hinggan Ling in China: an updated checklist. Biodiversity Data Journal 7: e32306. https://doi.org/10.3897/BDJ.7.e32306 Abstract Background This paper presents an updated list of tracheophytes of Xiao Hinggan Ling. The list includes 124 families, 503 genera and 1640 species (Containing subspecific units), of which 569 species (Containing subspecific units), 56 genera and 6 families represent first published records for Xiao Hinggan Ling. The aim of the present study is to document an updated checklist by reviewing the existing literature, browsing the website of National Specimen Information Infrastructure and additional data obtained in our research over the past ten years. This paper presents an updated list of tracheophytes of Xiao Hinggan Ling. The list includes 124 families, 503 genera and 1640 species (Containing subspecific units), of which 569 species (Containing subspecific units), 56 genera and 6 families represent first published records for Xiao Hinggan Ling. The aim of the present study is to document an updated checklist by reviewing the existing literature, browsing the website of National Specimen Information Infrastructure and additional data obtained in our research over the past ten years. -

Downloaded from Genbank on That Full Plastid Genomes Are Not Sufficient to Reject Al- February 28, 2012
Ruhfel et al. BMC Evolutionary Biology 2014, 14:23 http://www.biomedcentral.com/1471-2148/14/23 RESEARCH ARTICLE Open Access From algae to angiosperms–inferring the phylogeny of green plants (Viridiplantae) from 360 plastid genomes Brad R Ruhfel1*, Matthew A Gitzendanner2,3,4, Pamela S Soltis3,4, Douglas E Soltis2,3,4 and J Gordon Burleigh2,4 Abstract Background: Next-generation sequencing has provided a wealth of plastid genome sequence data from an increasingly diverse set of green plants (Viridiplantae). Although these data have helped resolve the phylogeny of numerous clades (e.g., green algae, angiosperms, and gymnosperms), their utility for inferring relationships across all green plants is uncertain. Viridiplantae originated 700-1500 million years ago and may comprise as many as 500,000 species. This clade represents a major source of photosynthetic carbon and contains an immense diversity of life forms, including some of the smallest and largest eukaryotes. Here we explore the limits and challenges of inferring a comprehensive green plant phylogeny from available complete or nearly complete plastid genome sequence data. Results: We assembled protein-coding sequence data for 78 genes from 360 diverse green plant taxa with complete or nearly complete plastid genome sequences available from GenBank. Phylogenetic analyses of the plastid data recovered well-supported backbone relationships and strong support for relationships that were not observed in previous analyses of major subclades within Viridiplantae. However, there also is evidence of systematic error in some analyses. In several instances we obtained strongly supported but conflicting topologies from analyses of nucleotides versus amino acid characters, and the considerable variation in GC content among lineages and within single genomes affected the phylogenetic placement of several taxa.