Cysteine-Rich Secretory Protein 4 Is an Inhibitor of Transient Receptor Potential M8 with a Role in Establishing Sperm Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unnatural Verticilide Enantiomer Inhibits Type 2 Ryanodine Receptor-Mediated Calcium Leak and Is Antiarrhythmic
Unnatural verticilide enantiomer inhibits type 2 ryanodine receptor-mediated calcium leak and is antiarrhythmic Suzanne M. Batistea,1, Daniel J. Blackwellb,1, Kyungsoo Kimb,1, Dmytro O. Kryshtalb, Nieves Gomez-Hurtadob, Robyn T. Rebbeckc, Razvan L. Corneac, Jeffrey N. Johnstona,2, and Bjorn C. Knollmannb,2 aDepartment of Chemistry, Vanderbilt University, Nashville, TN 37235; bDepartment of Medicine, Vanderbilt University Medical Center, Nashville, TN 37232; and cDepartment of Biochemistry, Molecular Biology, and Biophysics, University of Minnesota, Minneapolis, MN 55455 Edited by Dale L. Boger, The Scripps Research Institute, La Jolla, CA, and approved January 15, 2019 (received for review September 27, 2018) Ca2+ leak via ryanodine receptor type 2 (RyR2) can cause poten- heart diseases associated with both atrial and ventricular arrhyth- tially fatal arrhythmias in a variety of heart diseases and has also mia (9). Mutations in RyR2 and its binding partners, which increase + been implicated in neurodegenerative and seizure disorders, mak- SR Ca2 leak, cause primary atrial and ventricular arrhythmia ing RyR2 an attractive therapeutic target for drug development. syndromes such as catecholaminergic polymorphic ventricular Here we synthesized and investigated the fungal natural product tachycardia (CPVT), providing strong evidence for the mechanistic and known insect RyR antagonist (−)-verticilide and several conge- contribution of RyR2 to arrhythmia risk in humans (10). Further ners to determine their activity against mammalian RyR2. Although support comes from gene-targeted mouse models of CPVT, where + the cyclooligomeric depsipeptide natural product (−)-verticilide had catecholamine-induced spontaneous Ca2 release from the SR no effect, its nonnatural enantiomer [ent-(+)-verticilide] signifi- via RyR2 generates potentially fatal cardiac arrhythmias (11, 12). -

TRPM8 Activation by Menthol, Icilin, and Cold Is Differenially Modulated by Intracellular Ph
5364 • The Journal of Neuroscience, June 9, 2004 • 24(23):5364–5369 Cellular/Molecular TRPM8 Activation by Menthol, Icilin, and Cold Is Differentially Modulated by Intracellular pH David A. Andersson, Henry W. N. Chase, and Stuart Bevan Novartis Institute for Medical Sciences, London WC1E 6BN, United Kingdom TRPM8 is a nonselective cation channel activated by cold and the cooling compounds menthol and icilin (Peier et al., 2002). Here, we have used electrophysiology and the calcium-sensitive dye Fura-2 to study the effect of pH and interactions between temperature, pH, and the two chemical agonists menthol and icilin on TRPM8 expressed in Chinese hamster ovary cells. Menthol, icilin, and cold all evoked 2ϩ ϩ stimulus-dependent [Ca ]i responses in standard physiological solutions of pH 7.3. Increasing the extracellular [H ] from pH 7.3 to approximately pH 6 abolished responses to icilin and cold stimulation but did not affect responses to menthol. Icilin concentration– response curves were significantly shifted to the right when pH was lowered from 7.3 to 6.9, whereas those with menthol were unaltered in solutions of pH 6.1. When cells were exposed to solutions in the range of pH 8.1–6.5, the temperature threshold for activation was elevatedathigherpHanddepressedatlowerpH.Superfusingcellswithalowsubactivatingconcentrationoficilinormentholelevatedthe 2ϩ threshold for cold activation at pH 7.4, but cooling failed to evoke [Ca ]i responses at pH 6 in the presence of either agonist. In voltage-clamp experiments in which the intracellular pH was buffered to different levels, acidification reduced the current amplitude of icilin responses and shifted the threshold for cold activation to lower values with half-maximal inhibition at pH 7.2 and pH 7.6. -

Aquaporin Channels in the Heart—Physiology and Pathophysiology
International Journal of Molecular Sciences Review Aquaporin Channels in the Heart—Physiology and Pathophysiology Arie O. Verkerk 1,2,* , Elisabeth M. Lodder 2 and Ronald Wilders 1 1 Department of Medical Biology, Amsterdam University Medical Centers, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; [email protected] 2 Department of Experimental Cardiology, Amsterdam University Medical Centers, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; [email protected] * Correspondence: [email protected]; Tel.: +31-20-5664670 Received: 29 March 2019; Accepted: 23 April 2019; Published: 25 April 2019 Abstract: Mammalian aquaporins (AQPs) are transmembrane channels expressed in a large variety of cells and tissues throughout the body. They are known as water channels, but they also facilitate the transport of small solutes, gasses, and monovalent cations. To date, 13 different AQPs, encoded by the genes AQP0–AQP12, have been identified in mammals, which regulate various important biological functions in kidney, brain, lung, digestive system, eye, and skin. Consequently, dysfunction of AQPs is involved in a wide variety of disorders. AQPs are also present in the heart, even with a specific distribution pattern in cardiomyocytes, but whether their presence is essential for proper (electro)physiological cardiac function has not intensively been studied. This review summarizes recent findings and highlights the involvement of AQPs in normal and pathological cardiac function. We conclude that AQPs are at least implicated in proper cardiac water homeostasis and energy balance as well as heart failure and arsenic cardiotoxicity. However, this review also demonstrates that many effects of cardiac AQPs, especially on excitation-contraction coupling processes, are virtually unexplored. -

Supplemental Material
Supplemental Table B ARGs in alphabetical order Symbol Title 3 months 6 months 9 months 12 months 23 months ANOVA Direction Category 38597 septin 2 1557 ± 44 1555 ± 44 1579 ± 56 1655 ± 26 1691 ± 31 0.05219 up Intermediate 0610031j06rik kidney predominant protein NCU-G1 491 ± 6 504 ± 14 503 ± 11 527 ± 13 534 ± 12 0.04747 up Early Adult 1G5 vesicle-associated calmodulin-binding protein 662 ± 23 675 ± 17 629 ± 16 617 ± 20 583 ± 26 0.03129 down Intermediate A2m alpha-2-macroglobulin 262 ± 7 272 ± 8 244 ± 6 290 ± 7 353 ± 16 0.00000 up Midlife Aadat aminoadipate aminotransferase (synonym Kat2) 180 ± 5 201 ± 12 223 ± 7 244 ± 14 275 ± 7 0.00000 up Early Adult Abca2 ATP-binding cassette, sub-family A (ABC1), member 2 958 ± 28 1052 ± 58 1086 ± 36 1071 ± 44 1141 ± 41 0.05371 up Early Adult Abcb1a ATP-binding cassette, sub-family B (MDR/TAP), member 1A 136 ± 8 147 ± 6 147 ± 13 155 ± 9 185 ± 13 0.01272 up Midlife Acadl acetyl-Coenzyme A dehydrogenase, long-chain 423 ± 7 456 ± 11 478 ± 14 486 ± 13 512 ± 11 0.00003 up Early Adult Acadvl acyl-Coenzyme A dehydrogenase, very long chain 426 ± 14 414 ± 10 404 ± 13 411 ± 15 461 ± 10 0.01017 up Late Accn1 amiloride-sensitive cation channel 1, neuronal (degenerin) 242 ± 10 250 ± 9 237 ± 11 247 ± 14 212 ± 8 0.04972 down Late Actb actin, beta 12965 ± 310 13382 ± 170 13145 ± 273 13739 ± 303 14187 ± 269 0.01195 up Midlife Acvrinp1 activin receptor interacting protein 1 304 ± 18 285 ± 21 274 ± 13 297 ± 21 341 ± 14 0.03610 up Late Adk adenosine kinase 1828 ± 43 1920 ± 38 1922 ± 22 2048 ± 30 1949 ± 44 0.00797 up Early -

Single-Particle Cryo-EM of the Ryanodine Receptor Channel in an Aqueous Environment
Single-particle cryo-EM of the ryanodine receptor channel Eur J Transl Myol - Basic Appl Myol 2015; 25 (1): 35-48 Single-particle cryo-EM of the ryanodine receptor channel in an aqueous environment Mariah R. Baker, Guizhen Fan and Irina I. Serysheva Department of Biochemistry and Molecular Biology, The University of Texas Medical School at Houston, 6431 Fannin Street, Houston, TX 77030, USA Abstract Ryanodine receptors (RyRs) are tetrameric ligand-gated Ca2+ release channels that are responsible for the increase of cytosolic Ca2+ concentration leading to muscle contraction. Our current understanding of RyR channel gating and regulation is greatly limited due to the lack of a high-resolution structure of the channel protein. The enormous size and unwieldy shape of Ca2+ release channels make X-ray or NMR methods difficult to apply for high-resolution structural analysis of the full-length functional channel. Single-particle electron cryo- microscopy (cryo-EM) is one of the only effective techniques for the study of such a large integral membrane protein and its molecular interactions. Despite recent developments in cryo- EM technologies and break-through single-particle cryo-EM studies of ion channels, cryospecimen preparation, particularly the presence of detergent in the buffer, remains the main impediment to obtaining atomic-resolution structures of ion channels and a multitude of other integral membrane protein complexes. In this review we will discuss properties of several detergents that have been successfully utilized in cryo-EM studies of ion channels and the emergence of the detergent alternative amphipol to stabilize ion channels for structure- function characterization. Future structural studies of challenging specimen like ion channels are likely to be facilitated by cryo-EM amenable detergents or alternative surfactants. -
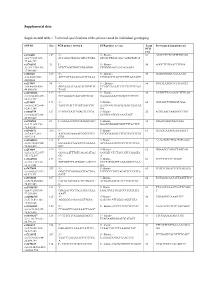
Supplemental Data Supplemental Table 1. Technical Specifications of the Primers Used for Individual Genotyping
Supplemental data Supplemental table 1. Technical specifications of the primers used for individual genotyping SNP ID Size PCR primer forward PCR primer reverse Temp Pyrosequencing primer(s) PCR (°C) rs776108 127 5’- 5’- Biotin- 61 ATACCTCTCATTTTGCAG chr3:77,825,927- ACCAGGCTAGGCATGCTATA GTCACTTAACAGCAGTGTGTCA 77,826,729 rs3746192 92 5’- 5’- Biotin- 56 AGGCTTGTAACCTGGA chr19:17,946,368- ATTCTAGGTGGCATGAGGG CTGGGGAGCAACAGAAGA 17,946,488 rs988147 169 5' - 5' - Biotine- 53 GCAGGGGGTAGAAATG chr6:45282108- AGCCATTAAAGAATTTCAAA TTGGATTTTATTCTTGTAATAGG 45282608 rs227849 94 5' - 5' - Biotine- 56 GGTTTAAGGTCTTTGCAT chr6:44,806,436- AGGAAAATAAACATGTGGTT TCTACCAATATTTTCTTTCGTAG 44,806,936 TAAG T rs10733833 127 5' - 5' - Biotine- 58 CATGTTTAAAACCTTTCAG chr10:68,418,227- GCCAAAACCAACAGTTCAT GAAAAAAATTGCACCTGTCTC 68,418,727 rs322609 197 5’- 5’-Biotin- 60 GGTAGCTGTGGGTGGA chr16:62,432,604- TAGTTGATTTTGCCAACCTG AAATGGGTGACAGAAGTAATAA 62,433,104 GA rs1884779 127 5’-TGGCTATTGGAGTTCTCA 5’-Biotin- 55 AGTGAATTAAGGGCTTGT chr20:45,857,969- CCATCCATCCCAAATAGT 45,858,469 rs4703908 83 5’- GAAAATGCCCAAGGTGAC 5’-Biotin- 52 GTGACAGTGGGCAAA chr5:71,802,353- GAATGTGGGTGTGTTTTACTCT 71,802,853 rs6946871 290 5’- 5’-Biotin- 61 GGAGGAAAGGAAAAGTT chr7:4,037,310- ATCAGATAAAATCGGCTTCT TCGGGAAGGTTTTTGTACTTTTG 4,037,810 GTG rs11865033 123 5’- 5’-Biotin- 61 AAAGTCTCTTCCTATGAGC chr16:78,082,945- AATAAACCAAGCCCTGAAAA ACTAAAATCCCCCTTTCCTCCA 78,083,445 GTC rs247004 170 5’- 5’-Biotin- 62 GGAAGCCAGACTAGCAG chr5:131,372,007- GGGGAATTTGTCAGAGATAG GGGATCCTCTACCATCCAAATA 131,372,507 GG -

Minding the Calcium Store: Ryanodine Receptor Activation As a Convergent Mechanism of PCB Toxicity
Pharmacology & Therapeutics 125 (2010) 260–285 Contents lists available at ScienceDirect Pharmacology & Therapeutics journal homepage: www.elsevier.com/locate/pharmthera Associate Editor: Carey Pope Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity Isaac N. Pessah ⁎, Gennady Cherednichenko, Pamela J. Lein Department of Molecular Biosciences, School of Veterinary Medicine, University of California, Davis, CA 95616, USA article info abstract Keywords: Chronic low-level polychlorinated biphenyl (PCB) exposures remain a significant public health concern since Ryanodine receptor (RyR) results from epidemiological studies indicate that PCB burden is associated with immune system Calcium-induced calcium release dysfunction, cardiovascular disease, and impairment of the developing nervous system. Of these various Calcium regulation adverse health effects, developmental neurotoxicity has emerged as a particularly vulnerable endpoint in Polychlorinated biphenyls PCB toxicity. Arguably the most pervasive biological effects of PCBs could be mediated by their ability to alter Triclosan fi 2+ Bastadins the spatial and temporal delity of Ca signals through one or more receptor-mediated processes. This Polybrominated diphenylethers review will focus on our current knowledge of the structure and function of ryanodine receptors (RyRs) in Developmental neurotoxicity muscle and nerve cells and how PCBs and related non-coplanar structures alter these functions. The Activity dependent plasticity molecular and cellular mechanisms by which non-coplanar PCBs and related structures alter local and global Ca2+ signaling properties and the possible short and long-term consequences of these perturbations on neurodevelopment and neurodegeneration are reviewed. © 2009 Elsevier Inc. All rights reserved. Contents 1. Introduction ............................................... 260 2. Ryanodine receptor macromolecular complexes: significance to polychlorinated biphenyl-mediated Ca2+ dysregulation . -

Comparative Genomic Analysis of Integral Membrane Transport Proteins in Ciliates
UC San Diego UC San Diego Previously Published Works Title Comparative genomic analysis of integral membrane transport proteins in ciliates. Permalink https://escholarship.org/uc/item/3g98s19z Journal The Journal of eukaryotic microbiology, 62(2) ISSN 1066-5234 Authors Kumar, Ujjwal Saier, Milton H Publication Date 2015-03-01 DOI 10.1111/jeu.12156 Peer reviewed eScholarship.org Powered by the California Digital Library University of California The Journal of Published by the International Society of Eukaryotic Microbiology Protistologists Journal of Eukaryotic Microbiology ISSN 1066-5234 ORIGINAL ARTICLE Comparative Genomic Analysis of Integral Membrane Transport Proteins in Ciliates Ujjwal Kumar & Milton H. Saier Jr Division of Biological Sciences, University of California at San Diego, La Jolla, California Keywords ABSTRACT Channels; evolution; genome analyses; secondary carriers. Integral membrane transport proteins homologous to those found in the Transporter Classification Database (TCDB; www.tcdb.org) were identified and Correspondence bioinformatically characterized by transporter class, family, and substrate speci- M. H. Saier Jr, Division of Biological ficity in three ciliates, Paramecium tetraurelia (Para), Tetrahymena thermophila Sciences, University of California at San (Tetra), and Ichthyophthirius multifiliis (Ich). In these three organisms, 1,326 of Diego, La Jolla, CA 92093-0116, USA 39,600 proteins (3.4%), 1,017 of 24,800 proteins (4.2%), and 504 out of 8,100 Telephone number: +858-534-4084; proteins (6.2%) integral membrane transport proteins were identified, respec- FAX number: +858-534-7108; tively. Thus, an inverse relationship was observed between the % transporters e-mail: [email protected] identified and the number of total proteins per genome reported. -

Snapshot: Mammalian TRP Channels David E
SnapShot: Mammalian TRP Channels David E. Clapham HHMI, Children’s Hospital, Department of Neurobiology, Harvard Medical School, Boston, MA 02115, USA TRP Activators Inhibitors Putative Interacting Proteins Proposed Functions Activation potentiated by PLC pathways Gd, La TRPC4, TRPC5, calmodulin, TRPC3, Homodimer is a purported stretch-sensitive ion channel; form C1 TRPP1, IP3Rs, caveolin-1, PMCA heteromeric ion channels with TRPC4 or TRPC5 in neurons -/- Pheromone receptor mechanism? Calmodulin, IP3R3, Enkurin, TRPC6 TRPC2 mice respond abnormally to urine-based olfactory C2 cues; pheromone sensing 2+ Diacylglycerol, [Ca ]I, activation potentiated BTP2, flufenamate, Gd, La TRPC1, calmodulin, PLCβ, PLCγ, IP3R, Potential role in vasoregulation and airway regulation C3 by PLC pathways RyR, SERCA, caveolin-1, αSNAP, NCX1 La (100 µM), calmidazolium, activation [Ca2+] , 2-APB, niflumic acid, TRPC1, TRPC5, calmodulin, PLCβ, TRPC4-/- mice have abnormalities in endothelial-based vessel C4 i potentiated by PLC pathways DIDS, La (mM) NHERF1, IP3R permeability La (100 µM), activation potentiated by PLC 2-APB, flufenamate, La (mM) TRPC1, TRPC4, calmodulin, PLCβ, No phenotype yet reported in TRPC5-/- mice; potentially C5 pathways, nitric oxide NHERF1/2, ZO-1, IP3R regulates growth cones and neurite extension 2+ Diacylglycerol, [Ca ]I, 20-HETE, activation 2-APB, amiloride, Cd, La, Gd Calmodulin, TRPC3, TRPC7, FKBP12 Missense mutation in human focal segmental glomerulo- C6 potentiated by PLC pathways sclerosis (FSGS); abnormal vasoregulation in TRPC6-/- -

Ca Signaling in Cardiac Fibroblasts and Fibrosis-Associated Heart
Journal of Cardiovascular Development and Disease Review Ca2+ Signaling in Cardiac Fibroblasts and Fibrosis-Associated Heart Diseases Jianlin Feng 1, Maria K. Armillei 1, Albert S. Yu 1, Bruce T. Liang 1, Loren W. Runnels 2,* and Lixia Yue 1,* 1 Calhoun Cardiology Center, Department of Cell Biology, University of Connecticut Health Center, Farmington, CT 06030, USA; [email protected] (J.F.); [email protected] (M.K.A.); [email protected] (A.S.Y.); [email protected] (B.T.L.) 2 Department of Pharmacology, Rutgers, Robert Wood Johnson Medical School, Piscataway, NJ 08854, USA * Correspondence: [email protected] (L.W.R.); [email protected] (L.Y.) Received: 11 August 2019; Accepted: 18 September 2019; Published: 23 September 2019 Abstract: Cardiac fibrosis is the excessive deposition of extracellular matrix proteins by cardiac fibroblasts and myofibroblasts, and is a hallmark feature of most heart diseases, including arrhythmia, hypertrophy, and heart failure. This maladaptive process occurs in response to a variety of stimuli, including myocardial injury, inflammation, and mechanical overload. There are multiple signaling pathways and various cell types that influence the fibrogenesis cascade. Fibroblasts and myofibroblasts are central effectors. Although it is clear that Ca2+ signaling plays a vital role in this pathological process, what contributes to Ca2+ signaling in fibroblasts and myofibroblasts is still not wholly understood, chiefly because of the large and diverse number of receptors, transporters, and ion channels that influence intracellular Ca2+ signaling. Intracellular Ca2+ signals are generated by Ca2+ release from intracellular Ca2+ stores and by Ca2+ entry through a multitude of Ca2+-permeable ion channels in the plasma membrane. -

Ion Channels 3 1
r r r Cell Signalling Biology Michael J. Berridge Module 3 Ion Channels 3 1 Module 3 Ion Channels Synopsis Ion channels have two main signalling functions: either they can generate second messengers or they can function as effectors by responding to such messengers. Their role in signal generation is mainly centred on the Ca2 + signalling pathway, which has a large number of Ca2+ entry channels and internal Ca2+ release channels, both of which contribute to the generation of Ca2 + signals. Ion channels are also important effectors in that they mediate the action of different intracellular signalling pathways. There are a large number of K+ channels and many of these function in different + aspects of cell signalling. The voltage-dependent K (KV) channels regulate membrane potential and + excitability. The inward rectifier K (Kir) channel family has a number of important groups of channels + + such as the G protein-gated inward rectifier K (GIRK) channels and the ATP-sensitive K (KATP) + + channels. The two-pore domain K (K2P) channels are responsible for the large background K current. Some of the actions of Ca2 + are carried out by Ca2+-sensitive K+ channels and Ca2+-sensitive Cl − channels. The latter are members of a large group of chloride channels and transporters with multiple functions. There is a large family of ATP-binding cassette (ABC) transporters some of which have a signalling role in that they extrude signalling components from the cell. One of the ABC transporters is the cystic − − fibrosis transmembrane conductance regulator (CFTR) that conducts anions (Cl and HCO3 )and contributes to the osmotic gradient for the parallel flow of water in various transporting epithelia. -

Disease Mutations in the Ryanodine Receptor N-Terminal Region Couple to a Mobile Intersubunit Interface
ARTICLE Received 1 Oct 2012 | Accepted 15 Jan 2013 | Published 19 Feb 2013 DOI: 10.1038/ncomms2501 OPEN Disease mutations in the ryanodine receptor N-terminal region couple to a mobile intersubunit interface Lynn Kimlicka1, Kelvin Lau1, Ching-Chieh Tung1 & Filip Van Petegem1 Ryanodine receptors are large channels that release Ca2 þ from the endoplasmic and sar- coplasmic reticulum. Hundreds of RyR mutations can cause cardiac and skeletal muscle disorders, yet detailed mechanisms explaining their effects have been lacking. Here we compare pseudo-atomic models and propose that channel opening coincides with widen- ing of a cytoplasmic vestibule formed by the N-terminal region, thus altering an interface targeted by 20 disease mutations. We solve crystal structures of several disease mutants that affect intrasubunit domain–domain interfaces. Mutations affecting intrasubunit ionic pairs alter relative domain orientations, and thus couple to surrounding interfaces. Buried disease mutations cause structural changes that also connect to the intersubunit contact area. These results suggest that the intersubunit contact region between N-terminal domains is a prime target for disease mutations, direct or indirect, and we present a model whereby ryanodine receptors and inositol-1,4,5-trisphosphate receptors are activated by altering domain arrangements in the N-terminal region. 1 Department of Biochemistry and Molecular Biology, Life Sciences Institute, University of British Columbia, Vancouver, British Columbia, Canada V6T 1Z3. Correspondence and requests for materials should be addressed to F.V.P. (email: fi[email protected]). NATURE COMMUNICATIONS | 4:1506 | DOI: 10.1038/ncomms2501 | www.nature.com/naturecommunications 1 & 2013 Macmillan Publishers Limited. All rights reserved.