114 Part 1308—Schedules of Controlled Substances
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cultural Rights in the United States: a Conflict of Aluesv
Minnesota Journal of Law & Inequality Volume 5 Issue 2 Article 3 June 1987 Cultural Rights in the United States: A Conflict of aluesV Sharon O'Brien Follow this and additional works at: https://lawandinequality.org/ Recommended Citation Sharon O'Brien, Cultural Rights in the United States: A Conflict of aluesV , 5(2) LAW & INEQ. 267 (1987). Available at: https://scholarship.law.umn.edu/lawineq/vol5/iss2/3 Minnesota Journal of Law & Inequality is published by the University of Minnesota Libraries Publishing. Cultural Rights in the United States: A Conflict of Values Sharon O'Brien* Introduction ................................................... 268 I. Historical Development of Minority Rights ............. 270 Historical Examples of Pluralistic Arrangements ..... 271 International Protection of Group Rights ............. 273 Leading National Examples of Group Rights Protection ............................................. 279 Twentieth-Century Pluralist Thought ................. 281 The United States and the Rights of Minorities ...... 283 Available Constitutional Mechanisms ................. 287 II. Am erican Indians ....................................... 290 Historical Background and Assimilation Efforts ...... 290 Cultural Protection ................................... 296 Freedom of Religion .................................. 298 III. Native Hawaiians ........................................ 308 Historical Background ................................. 308 Cultural Protection ................................... 314 Protection of the -

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

BOARD MEETING AGENDA Meeting Location: Portland State Office Building 800 NE Oregon Street, Portland, OR 97232 June 6-7, 2018 Updated 6.4.18
Oregon Board of Pharmacy BOARD MEETING AGENDA Meeting Location: Portland State Office Building 800 NE Oregon Street, Portland, OR 97232 June 6-7, 2018 Updated 6.4.18 The mission of the Oregon State Board of Pharmacy is to promote, preserve and protect the public health, safety and welfare by ensuring high standards in the practice of pharmacy and by regulating the quality, manufacture, sale and distribution of drugs. Wednesday, June 6, 2018 @ 8:30AM – Conference Rm A Thursday, June 7, 2018 @ 8:30AM – Conference Room A ≈ If special accommodations are needed for you to attend or participate in this Board Meeting, please contact Loretta Glenn at: (971) 673-0001. ≈ WEDNESDAY, JUNE 6, 2018 I. 8:30AM OPEN SESSION, Penny Reher, R.Ph, Presiding A. Roll Call B. Agenda Review and Approval Action Necessary II. Contested Case Deliberation pursuant to ORS 192.690(1) - Not Open to the Public III. EXECUTIVE SESSION – NOT OPEN TO THE PUBLIC, pursuant to ORS 676.175, ORS 192.660 (1) (2) (f) (k). A. Items for Consideration and Discussion: 1. Deliberation on Disciplinary Cases and Investigations 2. Personal Appearances 3. Deficiency Notifications 4. Case Review B. Employee Performance Review pursuant to ORS 192.660(2)(i). IV. OPEN SESSION - PUBLIC MAY ATTEND - At the conclusion of Executive Session, the Board may convene Open Session to begin some of the following scheduled agenda items - time permitting at approximately 3:30PM. V. Approve Consent Agenda* Action Necessary *Items listed under the consent agenda are considered to be routine agency matters and will be approved by a single motion of the Board without separate discussion. -

Journal of Museum Studies, Volume 8, Number 1 Journal of Museum Studies
THE UNIVERSITY OF OKLAHOMA COLLEGE OF LIBERAL STUDIES CLS Journal of Museum Studies, Volume 8, Number 1 Journal of Museum Studies e-Journal of the Museum Studies Program VOL 8 | NO 1 | DEC 2014 Foreword The Breadth of Natural History Research by Michael A. Mares Talking God and Father Peyote: Preliminary Quantification of Curator Religious Pluralism and Contemporary Diné Success in Life Science Natural History (Navajo) Art Collections by Daniel C. Swan and Dakota H. Stevens by Jessa L. Watters and Cameron D. Siler Edited by Michael A. Mares CLS Journal of Museum Studies, Volume 8, Number 1 CLS Journal of Museum Studies, Volume 8 Number 1 (Dec. 2014) http://jms.ou.edu CLS Journal of Museum Studies is currently published online by the College of Liberal Studies, MALS Museum Studies Program, the University of Oklahoma. Your use of the CLS Journal of Museum Studies archives indicates your acceptance of the Terms and Conditions of Use, available at http://jms.ou.edu. Museum professionals, students, and other readers are encouraged to distribute the articles published in this journal as widely as possible, to use them in classes, and to reprint them as needed. For commercial use of any of these articles Cover Photograph: Mother Earth, Father Sky and the Yeis (e.g., charging for articles, republishing figures, tables, text, etc.), Dancers, 2006. Jackie Black, Diné (Navajo), Red Valley, New permission must be obtained from the Editor. All questions Mexico. Acrylic on canvas. Sam Noble Museum. relating to the journal should be directed to the Editor. Journal Editor Publisher contact information available at http://jms.ou.edu. -

Native American Church
Native American Traditions Native American Church Native American Church Summary: The Native American Church of Jesus Christ is a spiritual movement that integrates the teachings of Christian life with the spiritual and ethical traditions of various Native cultures. A central component of the Native American Church is the sacramental ingestion of peyote during peyote meetings. In 1990, the U.S. Supreme Court has ruled that the ritual ingestion of peyote is not a legally protected practice, despite its religious significance. The Native American Church of Jesus Christ is the full name of a widespread spiritual movement that integrates the teachings of Christian life with the spiritual and ethical traditions of various Native cultures regarding the sacramental ingestion of peyote. For some participants, the Christian context is important, but for others the Native American Church is much more an affirmation of Indian religious identity. While the visionary effect of the peyote cactus button has captured the imagination of many psychedelic spiritual seekers, it is critical that those who would understand the ceremonial use of peyote situate this sacramental tradition within the rigorous ethical way of life taught by the Native American Church. Many church members believe that a narrow perception of peyote as simply an hallucinogen or “drug” informed the U.S. Supreme Court’s 1990 decision in Employment Division of Oregon v. Smith. In the Smith case, two Native Americans had been fired from their state jobs in Oregon because they participated in a peyote ceremony of the Native American Church. Peyote had been listed as a proscribed drug in the state. -
Prezentace Aplikace Powerpoint
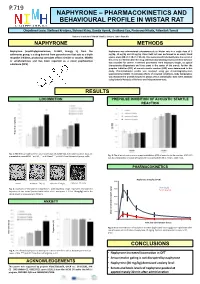
P.719 NAPHYRONE – PHARMACOKINETICS AND BEHAVIOURAL PROFILE IN WISTAR RAT Olejíková Lucie, Štefková Kristýa, Šíchová Klára, Dada Hyek, Lhotková Eva, Piterová Nikola, Páleíček Toáš National Institute of Mental Health, Klecany, Czech Republic NAPHYRONE METHODS Naphyrone (naphthylpyrovalerone, O-2482, Energy 1), from the Naphyrone was administered subcutaneously to Wistar rats in a single dose of 5 cathinones group, is a drug derived from pyrovalerone that acts as a triple mg/kg, 10 mg/kg and 20 mg/kg. Open field test was performed in an empty black reuptake inhibitor, producing stimulant effects similar to cocaine, MDMA square arena (68 c × 68 c × 30 c). Rats were placed individually into the center of or amphetamines and has been reported as a novel psychoactive the arena 5 or 40 i after the drug administration (testing-onset) and their behavior was recorded for 30 i. Examined parameters were trajectory length, its spatial substance (NPS). characteristic (thigmotaxis and time spent in the center of the arena). Further the prepulse inhibition (PPI) of acoustic startle reaction (ASR) were determined in this study. Pharmacokinetic profile was analyzed using gas chromatography-mass spectrometry (GCMS). To simulate effects of crowded conditions, body temperature was monitored in animals housed in groups versus individually. Data were analyzed using factorial Analysis of Variance and independent t-tests. RESULTS LOCOMOTION PREPULSE INHIBITION OF ACOUSTIC STARTLE REACTION lenght (m) Trajectory Fig. 1: The effect of naphyrone on total locomotion 15 and 60 min after administration. Data are Fig. 3: The effect of naphyrone on prepulse inhibition (PPI) of acoustic startle reaction (ASR). PPI presented as mean±SEM. -

A Review of a Culture's Catalyst: Historical Encounters with Peyote and the Native American Church in Canada
IK: Other Ways of Knowing Book Review A Review of A Culture’s Catalyst: Historical Encounters with Peyote and the Native American Church in Canada Book Review by Kevin Feeney PhD. JD, Central Washington University A Culture’s Catalyst: Historical Encounters with Peyote and the Native American Church in Canada by Kahan, Fannie, edited by Erika Dyck. 2016. Winnipeg, MB: University of Manitoba Press. 130 pp. Paperback $27.95. ISBN 978-0887558146 doi 10.18113/P8ik360543 Fannie Kahan’s new book, A Culture’s Catalyst, provides a novel glimpse into an era when psychedelic research was at its peak, and when social fears about psychedelics were limited to the imagined orgies of peyote cults. Kahan’s original manuscript was completed back in 1963, but failed efforts at finding a publisher left the book gathering dust in the archives of the author’s brother, Abram Hoffer. The manuscript remained buried in papers until it was uncovered by historian Erika Dyck during investigations on the history of psychedelic research in Canada. Dyck has brought this manuscript to light by editing it for clarity and uniformity, and providing an in-depth introduction contextualizing Kahan’s work within the political landscape of the 1950s and early 1960s. Kahan’s book is largely built around a Native American Church peyote ceremony held during 1956 in North Battleford, Sasketchewan. A handful of White scientists attended the ceremony, including book contributors Duncan Blewett, Abram Hoffer, Humphry Osmond, and Teodoro Weckowicz. Kahan, who is a journalist by training, has assembled and presented what might best be characterized as a defense of peyotism, the religious use of peyote, with a text that veers between reporting and editorializing. -

Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected]
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 4-25-2018 Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected] DOI: 10.25148/etd.FIDC006565 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Chemistry Commons Recommended Citation Seither, Joshua Zolton, "Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances" (2018). FIU Electronic Theses and Dissertations. 3823. https://digitalcommons.fiu.edu/etd/3823 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida APPLICATION OF HIGH RESOLUTION MASS SPECTROMETRY FOR THE SCREENING AND CONFIRMATION OF NOVEL PSYCHOACTIVE SUBSTANCES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Joshua Zolton Seither 2018 To: Dean Michael R. Heithaus College of Arts, Sciences and Education This dissertation, written by Joshua Zolton Seither, and entitled Application of High- Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Piero Gardinali _______________________________________ Bruce McCord _______________________________________ DeEtta Mills _______________________________________ Stanislaw Wnuk _______________________________________ Anthony DeCaprio, Major Professor Date of Defense: April 25, 2018 The dissertation of Joshua Zolton Seither is approved. -

Expansion of a Cheminformatic Database of Spectral Data for Forensic Chemists and Toxicologists
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Expansion of a Cheminformatic Database of Spectral Data for Forensic Chemists and Toxicologists Author(s): Peter Stout, Katherine Moore, Megan Grabenauer, Jeri Ropero-Miller Document No.: 241444 Date Received: March 2013 Award Number: 2010-DN-BX-K177 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Award Number: 2010-DN-BX-K177 July 16, 2012 Expansion of a Cheminformatic Database of Spectral Data for Forensic Chemists and Toxicologists Final Report Authors: Peter Stout Katherine Moore Megan Grabenauer Jeri Ropero-Miller This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Expansion of a Cheminformatic Database of Spectral Data for Forensic Chemists and Toxicologists Table of Contents Abstract ........................................................................................................................................... 1 Executive Summary ....................................................................................................................... -

A Long Strange Trip: Latin America's Contribution to World Drug Culture
e l e v e n A Long Strange Trip latin america’s contribution to world drug culture Paul Gootenberg Perhaps it’s risky, even foolhardy, for a historian to suggest that drugs are one of Latin America’s enduring contributions to world culture. Today, “drugs” mostly conjures up cocaine, meth, addiction, corruption, traf- fickers, DEA drug wars, and the unfathomably brutal bloodletting along the northern borders of Mexico. It also suggests the unfortunate tendency of (North) Americans to blame their intemperate desires for consuming drugs on evil foreigners, the Pablo Escobars and Chapo Guzmáns, or wily “cartels” (a term that makes specialists cringe). It also serves to sweep under the rug the complicity of our institutions and badly conceived drug laws and policies— namely, global drug prohibition—in igniting the kinds of mayhem that have afflicted places like Colombia, Peru, and Mexico in recent decades. So, here, I’d like to step back and suggest as a corrective a few less sensationalistic, less ahistorical, less U.S.-centric points: the richer, longer cornucopia of mind- altering goods the Americas have offered the world; some of the changing historical entanglements of these drugs with global culture; and, most recently, promising new shifts, coming from the Global “South,” on how to rethink our bellicose current posture on illicit drugs. This long trip through hemispheric drugs is rich in historical ironies. psychedelic civilizations Thousands of years ago, prehistoric Americans invented what the ethnobota- nist Winston LaBarre termed the “American drug complex,” the world’s wid- est and wildest menu of psychotropic drugs. -

Neuropsychedelia
Neuropsychedelia The Revival of Hallucinogen Research since the Decade of the Brain Nicolas Langlitz UNIVERSITY OF CALIFORNIA PRESS Berkeley • Los Angeles • London Contents. University of California Press, one of the most distinguished university presses in the United States, enriches lives around the world by advancing scholarship in the humanities, social sciences, and natural sciences. Its activities are supported by the UC Press Foundation and by philanthropic contributions from individuals and institutions. For more information, visit www.ucpress.edu. University of California Press Berkeley and Los Angeles, California University of California Press, Ltd. London, England © 2013 by The Regents of the University of California Acknowledgments Vtl Library of Congress Cataloging-in-Publication Data Introduction: Neuropsychopharmacology Langlitz, Nicolas, 1975-. Neuropsychedelia : the revival of hallucinogen as Spiritual Technology I research since the decade of the brain I Nicolas Langlitz. 1. Psychedelic Revival p. cm. Includes bibliographical references and index. 2. Swiss Psilocybin and US Dollars 53 ISBN 978-0-520-27481-5 (cloth: alk. paper) ISBN 978-0-520-27482-2 (pbk. : alk. paper) 3. The Varieties of Psychedelic Lab Experience I. Hallucinogenic drugs-Research. 2. Neuropsychopharmacology. 3. Hallucinogenic 4. Enacting Experimental Psychoses 2 drugs and religious experience. 1. Title. I3 BF209·H34L36 2013 - I54·4-dc23 5. Between Animality and Divinity I66 2012022916 6. Mystic Materialism 2°4 Manufactured in the United States of America Conclusion: Fieldwork in Perennial Philosophy 243 21 20 19 18 17 16 15 14 13 10 9 8 7 6 5 4 3 2 In keeping with a commitment to support Notes environmentally responsible and sustainable printing practices, UC Press has printed this book on 50-pound Bibliography Enterprise, a 30% post-consumer-waste, recycled, Index deinked fiber that is processed chlorine-free. -

Appendix-2Final.Pdf 663.7 KB
North West ‘Through the Gate Substance Misuse Services’ Drug Testing Project Appendix 2 – Analytical methodologies Overview Urine samples were analysed using three methodologies. The first methodology (General Screen) was designed to cover a wide range of analytes (drugs) and was used for all analytes other than the synthetic cannabinoid receptor agonists (SCRAs). The analyte coverage included a broad range of commonly prescribed drugs including over the counter medications, commonly misused drugs and metabolites of many of the compounds too. This approach provided a very powerful drug screening tool to investigate drug use/misuse before and whilst in prison. The second methodology (SCRA Screen) was specifically designed for SCRAs and targets only those compounds. This was a very sensitive methodology with a method capability of sub 100pg/ml for over 600 SCRAs and their metabolites. Both methodologies utilised full scan high resolution accurate mass LCMS technologies that allowed a non-targeted approach to data acquisition and the ability to retrospectively review data. The non-targeted approach to data acquisition effectively means that the analyte coverage of the data acquisition was unlimited. The only limiting factors were related to the chemical nature of the analyte being looked for. The analyte must extract in the sample preparation process; it must chromatograph and it must ionise under the conditions used by the mass spectrometer interface. The final limiting factor was presence in the data processing database. The subsequent study of negative MDT samples across the North West and London and the South East used a GCMS methodology for anabolic steroids in addition to the General and SCRA screens.