Expansion of a Cheminformatic Database of Spectral Data for Forensic Chemists and Toxicologists
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antinociceptive Effects of Monoamine Reuptake Inhibitors in Assays of Pain-Stimulated and Pain-Depressed Behaviors
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2012 Antinociceptive Effects of Monoamine Reuptake Inhibitors in Assays of Pain-Stimulated and Pain-Depressed Behaviors Marisa Rosenberg Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Medical Pharmacology Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/2715 This Thesis is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. ANTINOCICEPTIVE EFFECTS OF MONOAMINE REUPTAKE INHIBITORS IN ASSAYS OF PAIN-STIMULATED AND PAIN-DEPRESSED BEHAVIOR A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science at Virginia Commonwealth University By Marisa B. Rosenberg Bachelor of Science, Temple University, 2008 Advisor: Sidney Stevens Negus, Ph.D. Professor, Department of Pharmacology/Toxicology Virginia Commonwealth University Richmond, VA May, 2012 Acknowledgement First and foremost, I’d like to thank my advisor Dr. Steven Negus, whose unwavering support, guidance and patience throughout my graduate career has helped me become the scientist I am today. His dedication to education, learning and the scientific process has instilled in me a quest for knowledge that I will continue to pursue in life. His thoroughness, attention to detail and understanding of pharmacology has been exemplary to a young person like me just starting out in the field of science. I’d also like to thank all of my committee members (Drs. -

Dopamine Reuptake Transporter (DAT) “Inverse Agonism” E Anovel 66 2 67 3 Hypothesis to Explain the Enigmatic Pharmacology of Cocaine 68 4 * 69 5 Q5 David J
NP5526_proof ■ 24 June 2014 ■ 1/22 Neuropharmacology xxx (2014) 1e22 55 Contents lists available at ScienceDirect 56 57 Neuropharmacology 58 59 60 journal homepage: www.elsevier.com/locate/neuropharm 61 62 63 64 65 1 Dopamine reuptake transporter (DAT) “inverse agonism” e Anovel 66 2 67 3 hypothesis to explain the enigmatic pharmacology of cocaine 68 4 * 69 5 Q5 David J. Heal , Jane Gosden, Sharon L. Smith 70 6 71 RenaSci Limited, BioCity, Pennyfoot Street, Nottingham NG1 1GF, UK 7 72 8 73 9 article info abstract 74 10 75 11 76 Article history: The long held view is cocaine's pharmacological effects are mediated by monoamine reuptake inhibition. 12 Available online xxx However, drugs with rapid brain penetration like sibutramine, bupropion, mazindol and tesofensine, 77 13 which are equal to or more potent than cocaine as dopamine reuptake inhibitors, produce no discernable 78 14 Keywords: subjective effects such as drug “highs” or euphoria in drug-experienced human volunteers. Moreover 79 15 Cocaine they are dysphoric and aversive when given at high doses. In vivo experiments in animals demonstrate 80 16 Dopamine reuptake inhibitor that cocaine's monoaminergic pharmacology is profoundly different from that of other prescribed 81 Dopamine releasing agent 17 monoamine reuptake inhibitors, with the exception of methylphenidate. These findings led us to 82 Dopamine transporter fi 18 Inverse agonist conclude that the highly unusual, stimulant pro le of cocaine and related compounds, eg methylphe- 83 19 DAT inverse agonist nidate, is not mediated by monoamine reuptake inhibition alone. 84 We describe the experimental findings which suggest cocaine serves as a negative allosteric 20 Novel mechanism 85 21 modulator to alter the function of the dopamine reuptake transporter (DAT) and reverse its direction of fi 86 22 transport. -

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

BOARD MEETING AGENDA Meeting Location: Portland State Office Building 800 NE Oregon Street, Portland, OR 97232 June 6-7, 2018 Updated 6.4.18
Oregon Board of Pharmacy BOARD MEETING AGENDA Meeting Location: Portland State Office Building 800 NE Oregon Street, Portland, OR 97232 June 6-7, 2018 Updated 6.4.18 The mission of the Oregon State Board of Pharmacy is to promote, preserve and protect the public health, safety and welfare by ensuring high standards in the practice of pharmacy and by regulating the quality, manufacture, sale and distribution of drugs. Wednesday, June 6, 2018 @ 8:30AM – Conference Rm A Thursday, June 7, 2018 @ 8:30AM – Conference Room A ≈ If special accommodations are needed for you to attend or participate in this Board Meeting, please contact Loretta Glenn at: (971) 673-0001. ≈ WEDNESDAY, JUNE 6, 2018 I. 8:30AM OPEN SESSION, Penny Reher, R.Ph, Presiding A. Roll Call B. Agenda Review and Approval Action Necessary II. Contested Case Deliberation pursuant to ORS 192.690(1) - Not Open to the Public III. EXECUTIVE SESSION – NOT OPEN TO THE PUBLIC, pursuant to ORS 676.175, ORS 192.660 (1) (2) (f) (k). A. Items for Consideration and Discussion: 1. Deliberation on Disciplinary Cases and Investigations 2. Personal Appearances 3. Deficiency Notifications 4. Case Review B. Employee Performance Review pursuant to ORS 192.660(2)(i). IV. OPEN SESSION - PUBLIC MAY ATTEND - At the conclusion of Executive Session, the Board may convene Open Session to begin some of the following scheduled agenda items - time permitting at approximately 3:30PM. V. Approve Consent Agenda* Action Necessary *Items listed under the consent agenda are considered to be routine agency matters and will be approved by a single motion of the Board without separate discussion. -
Prezentace Aplikace Powerpoint
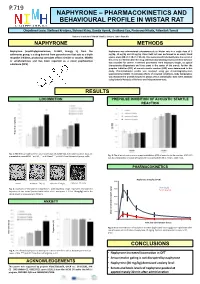
P.719 NAPHYRONE – PHARMACOKINETICS AND BEHAVIOURAL PROFILE IN WISTAR RAT Olejíková Lucie, Štefková Kristýa, Šíchová Klára, Dada Hyek, Lhotková Eva, Piterová Nikola, Páleíček Toáš National Institute of Mental Health, Klecany, Czech Republic NAPHYRONE METHODS Naphyrone (naphthylpyrovalerone, O-2482, Energy 1), from the Naphyrone was administered subcutaneously to Wistar rats in a single dose of 5 cathinones group, is a drug derived from pyrovalerone that acts as a triple mg/kg, 10 mg/kg and 20 mg/kg. Open field test was performed in an empty black reuptake inhibitor, producing stimulant effects similar to cocaine, MDMA square arena (68 c × 68 c × 30 c). Rats were placed individually into the center of or amphetamines and has been reported as a novel psychoactive the arena 5 or 40 i after the drug administration (testing-onset) and their behavior was recorded for 30 i. Examined parameters were trajectory length, its spatial substance (NPS). characteristic (thigmotaxis and time spent in the center of the arena). Further the prepulse inhibition (PPI) of acoustic startle reaction (ASR) were determined in this study. Pharmacokinetic profile was analyzed using gas chromatography-mass spectrometry (GCMS). To simulate effects of crowded conditions, body temperature was monitored in animals housed in groups versus individually. Data were analyzed using factorial Analysis of Variance and independent t-tests. RESULTS LOCOMOTION PREPULSE INHIBITION OF ACOUSTIC STARTLE REACTION lenght (m) Trajectory Fig. 1: The effect of naphyrone on total locomotion 15 and 60 min after administration. Data are Fig. 3: The effect of naphyrone on prepulse inhibition (PPI) of acoustic startle reaction (ASR). PPI presented as mean±SEM. -

Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected]
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 4-25-2018 Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected] DOI: 10.25148/etd.FIDC006565 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Chemistry Commons Recommended Citation Seither, Joshua Zolton, "Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances" (2018). FIU Electronic Theses and Dissertations. 3823. https://digitalcommons.fiu.edu/etd/3823 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida APPLICATION OF HIGH RESOLUTION MASS SPECTROMETRY FOR THE SCREENING AND CONFIRMATION OF NOVEL PSYCHOACTIVE SUBSTANCES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Joshua Zolton Seither 2018 To: Dean Michael R. Heithaus College of Arts, Sciences and Education This dissertation, written by Joshua Zolton Seither, and entitled Application of High- Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Piero Gardinali _______________________________________ Bruce McCord _______________________________________ DeEtta Mills _______________________________________ Stanislaw Wnuk _______________________________________ Anthony DeCaprio, Major Professor Date of Defense: April 25, 2018 The dissertation of Joshua Zolton Seither is approved. -

Appendix-2Final.Pdf 663.7 KB
North West ‘Through the Gate Substance Misuse Services’ Drug Testing Project Appendix 2 – Analytical methodologies Overview Urine samples were analysed using three methodologies. The first methodology (General Screen) was designed to cover a wide range of analytes (drugs) and was used for all analytes other than the synthetic cannabinoid receptor agonists (SCRAs). The analyte coverage included a broad range of commonly prescribed drugs including over the counter medications, commonly misused drugs and metabolites of many of the compounds too. This approach provided a very powerful drug screening tool to investigate drug use/misuse before and whilst in prison. The second methodology (SCRA Screen) was specifically designed for SCRAs and targets only those compounds. This was a very sensitive methodology with a method capability of sub 100pg/ml for over 600 SCRAs and their metabolites. Both methodologies utilised full scan high resolution accurate mass LCMS technologies that allowed a non-targeted approach to data acquisition and the ability to retrospectively review data. The non-targeted approach to data acquisition effectively means that the analyte coverage of the data acquisition was unlimited. The only limiting factors were related to the chemical nature of the analyte being looked for. The analyte must extract in the sample preparation process; it must chromatograph and it must ionise under the conditions used by the mass spectrometer interface. The final limiting factor was presence in the data processing database. The subsequent study of negative MDT samples across the North West and London and the South East used a GCMS methodology for anabolic steroids in addition to the General and SCRA screens. -

Director's Report 2/01
Director's Report 2/01 Director's Report to the National Advisory Council on Drug Abuse February, 2001 Index Research Findings Basic Research Behavioral Research Treatment Research and Development Research on AIDS and Other Medical Consequences of Drug Abuse Epidemiology, Etiology and Prevention Research Services Research Intramural Research Program Activities Extramural Policy and Review Activities Congressional Affairs International Activities Meetings and Conferences Media and Education Activities Planned Meetings Publications Staff Highlights Grantee Honors [Office of Director] [First Report Section] Archive Home | Accessibility | Privacy | FOIA (NIH) | Current NIDA Home Page The National Institute on Drug Abuse (NIDA) is part of the National Institutes of Health (NIH) , a component of the U.S. Department of Health and Human Services. Questions? _ See our Contact Information. https://archives.drugabuse.gov/DirReports/DirRep201/DirectorRepIndex.html[11/17/16, 9:48:57 PM] Director's Report 2/01 - Basic Research Director's Report to the National Advisory Council on Drug Abuse February, 2001 Research Findings Basic Research Role for GDNF in Biochemical and Behavioral Adaptations to Drugs of Abuse Drs. David Russell and Eric Nestler and their research team at the Yale University examined a role for Glial-Derived Neurotrophic Factor (GDNF) in adaptations to drugs of abuse. Infusion of GDNF into the ventral tegmental area (VTA), a dopaminergic brain region important for addiction, blocks certain biochemical adaptations to chronic cocaine or morphine as well as the rewarding effects of cocaine. Conversely, responses to cocaine are enhanced in rats by intra-VTA infusion of an anti-GDNF antibody and in mice heterozygous for a null mutation in the GDNF gene. -

Schedules of Controlled Substances (.Pdf)
PURSUANT TO THE TEXAS CONTROLLED SUBSTANCES ACT, HEALTH AND SAFETY CODE, CHAPTER 481, THESE SCHEDULES SUPERCEDE PREVIOUS SCHEDULES AND CONTAIN THE MOST CURRENT VERSION OF THE SCHEDULES OF ALL CONTROLLED SUBSTANCES FROM THE PREVIOUS SCHEDULES AND MODIFICATIONS. This annual publication of the Texas Schedules of Controlled Substances was signed by John Hellerstedt, M.D., Commissioner of Health, and will take effect 21 days following publication of this notice in the Texas Register. Changes to the schedules are designated by an asterisk (*). Additional information can be obtained by contacting the Department of State Health Services, Drugs and Medical Devices Unit, P.O. Box 149347, Austin, Texas 78714-9347. The telephone number is (512) 834-6755 and the website address is http://www.dshs.texas.gov/dmd. SCHEDULES Nomenclature: Controlled substances listed in these schedules are included by whatever official, common, usual, chemical, or trade name they may be designated. SCHEDULE I Schedule I consists of: -Schedule I opiates The following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters, and ethers, unless specifically excepted, if the existence of these isomers, esters, ethers, and salts are possible within the specific chemical designation: (1) Acetyl-α-methylfentanyl (N-[1-(1-methyl-2-phenethyl)-4-piperidinyl]-N- phenylacetamide); (2) Acetylmethadol; (3) Acetyl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylacetamide); (4) Acryl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylacrylamide) (Other name: -

This List Was Originally Published in Virginia Register Volume 8, Issue 18
In accordance with 6 VAC 40-30, the Regulations for the Approval of Field Tests for Detection of Drugs, and under the authority of the Code of Virginia, the following field tests for detection of drugs are approved field tests: O D V INCORPORATED 13386 INTERNATIONAL PARKWAY JACKSONVILLE, FLORIDA 32218-2383 ODV NarcoPouch Drug or Drug Type: Manufacturer’s Field Test: Heroin 902 – Marquis Reagent Amphetamine 902 – Marquis Reagent Methamphetamine 902 – Marquis Reagent 3,4–Methylenedioxymethamphetamine (MDMA) 902 – Marquis Reagent Cocaine Hydrochloride 904 or 904B – Cocaine HCl and Base Reagent Cocaine Base 904 or 904B – Cocaine HCl and Base Reagent Barbiturates 905 – Dille-Koppanyi Reagent Lysergic Acid Diethylamide (LSD) 907 – Ehrlich’s (Modified) Reagent Marijuana 908 – Duquenois – Levine Reagent Hashish Oil 908 – Duquenois – Levine Reagent Marijuana 909 – K N Reagent Hashish Oil 909 – K N Reagent Phencyclidine (PCP) 914 – PCP Methaqualone Reagent Heroin 922 – Opiates Reagent Methamphetamine 923 – Methamphetamine/Ecstasy Reagent 3,4–Methylenedioxymethamphetamine (MDMA) 923 – Methamphetamine/Ecstasy Reagent Heroin 924 – Mecke’s (Modified) Reagent Diazepam 925 – Valium/Ketamine Reagent Ketamine 925 – Valium/Ketamine Reagent Ephedrine 927 – Ephedrine Reagent gamma – Hydroxybutyrate (GHB) 928 – GHB Reagent ODV NarcoTest Drug or Drug Type: Manufacturer’s Field Test: Heroin 7602 – Marquis Reagent Amphetamine 7602 – Marquis Reagent Methamphetamine 7602 – Marquis Reagent 3,4–Methylenedioxymethamphetamine (MDMA) 7602 – Marquis Reagent Barbiturates -

Drugs and New Potentially Dangerous Chemical Substances, with a Brief Review of the Problem
INTERNATIONAL JOURNAL OF ENVIRONMENTAL & SCIENCE EDUCATION 2016, VOL. 11, NO. 14, 6697-6703 OPEN ACCESS Approach to Classifying “Design” Drugs and New Potentially Dangerous Chemical Substances, With a Brief Review of the Problem Azat R. Asadullina, Elena Kh. Galeevab, Elvina A. Achmetovaa and Ivan V. Nikolaevb aBashkir State Medical University of the Ministry of Health of the Russian Federation, Ufa, RUSSIA; bRepublican Narcological Dispensary No.1 of the Ministry of Health of the Republic of Bashkortostan, Ufa, RUSSIA ABSTRACT The urgency of this study has become vivid in the light of the growing problem of prevalence and bBPHFuse Republican of new synthetic Narcological drug types. DispensaryLately there has No.1 been of a thetendency Ministry of expanding of Health the rangeof the of psychologically active substances (PAS) used by addicts with the purpose of their illegal taking. RepublicThe aim of Bashkortostan,of this research is anPushkin attempt str.,of systematizing 119/1, Ufa, and RB. classifying “design” drugs according to their chemical structure, neurochemical mechanisms of action and clinical manifestations. As a result, we have found that they can be divided into ten big groups. This classification will allow to better arrange new clinical phenomenology in modern addictology. This paper would be useful for psychiatrists, experts in narcology, as well as for personnel of institutions and agencies engaged in anti-drug activity. KEYWORDS ARTICLE HISTORY Opiates, cannabinoids, cathinones, tryptamine, Received 20 April 2016 cocaine, pregabalin, new “design” drugs, Revised 28 May 2016 classification Accepted 29 Мау 2016 Introduction Urgency of the problem Recently, the sphere of illegal turnover of narcotic substances has shown an apparent trend of producing the so-called “design drugs” (DN): new potentially dangerous psychologically active substances (NPDPAS) obtained by means of chemical synthesis, possessing a high narcotic effect and manufactured with the CORRESPONDENCE Azat R. -

World of Cognitive Enhancers
ORIGINAL RESEARCH published: 11 September 2020 doi: 10.3389/fpsyt.2020.546796 The Psychonauts’ World of Cognitive Enhancers Flavia Napoletano 1,2, Fabrizio Schifano 2*, John Martin Corkery 2, Amira Guirguis 2,3, Davide Arillotta 2,4, Caroline Zangani 2,5 and Alessandro Vento 6,7,8 1 Department of Mental Health, Homerton University Hospital, East London Foundation Trust, London, United Kingdom, 2 Psychopharmacology, Drug Misuse, and Novel Psychoactive Substances Research Unit, School of Life and Medical Sciences, University of Hertfordshire, Hatfield, United Kingdom, 3 Swansea University Medical School, Institute of Life Sciences 2, Swansea University, Swansea, United Kingdom, 4 Psychiatry Unit, Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy, 5 Department of Health Sciences, University of Milan, Milan, Italy, 6 Department of Mental Health, Addictions’ Observatory (ODDPSS), Rome, Italy, 7 Department of Mental Health, Guglielmo Marconi” University, Rome, Italy, 8 Department of Mental Health, ASL Roma 2, Rome, Italy Background: There is growing availability of novel psychoactive substances (NPS), including cognitive enhancers (CEs) which can be used in the treatment of certain mental health disorders. While treating cognitive deficit symptoms in neuropsychiatric or neurodegenerative disorders using CEs might have significant benefits for patients, the increasing recreational use of these substances by healthy individuals raises many clinical, medico-legal, and ethical issues. Moreover, it has become very challenging for clinicians to Edited by: keep up-to-date with CEs currently available as comprehensive official lists do not exist. Simona Pichini, Methods: Using a web crawler (NPSfinder®), the present study aimed at assessing National Institute of Health (ISS), Italy Reviewed by: psychonaut fora/platforms to better understand the online situation regarding CEs.