Safety Data Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Common Name: P-TOLUENE SULFONIC ACID HAZARD
Common Name: p-TOLUENE SULFONIC ACID CAS Number: 104-15-4 DOT Number: UN 2583 (solid, with more than 5% free Sulfuric Acid) UN 2585 (solid, with less than 5% free RTK Substance number: 1870 Sulfuric Acid) Date: October 1996 Revision: May 2003 ------------------------------------------------------------------------- ------------------------------------------------------------------------- HAZARD SUMMARY WORKPLACE EXPOSURE LIMITS * p-Toluene Sulfonic Acid can affect you when breathed in. No occupational exposure limits have been established for * p-Toluene Sulfonic Acid is a CORROSIVE CHEMICAL p-Toluene Sulfonic Acid. This does not mean that this and contact can cause severe skin and eye irritation and substance is not harmful. Safe work practices should always burns. be followed. * Exposure to p-Toluene Sulfonic Acid can irritate the nose, throat and lungs causing burning, dryness and WAYS OF REDUCING EXPOSURE coughing. * Where possible, enclose operations and use local exhaust ventilation at the site of chemical release. If local exhaust IDENTIFICATION ventilation or enclosure is not used, respirators should be p-Toluene Sulfonic Acid is a colorless, clear, or dark-colored worn. liquid or a colorless, crystalline (sand-like) material. It is used * Wear protective work clothing. to make dyes, drugs and other chemicals. * Wash thoroughly immediately after exposure to p- Toluene Sulfonic Acid and at the end of the workshift. REASON FOR CITATION * Post hazard and warning information in the work area. In * p-Toluene Sulfonic Acid is on the Hazardous Substance addition, as part of an ongoing education and training List because it is cited by DOT and NFPA. effort, communicate all information on the health and * This chemical is on the Special Health Hazard Substance safety hazards of p-Toluene Sulfonic Acid to potentially List because it is CORROSIVE. -

Structural Determination of Subsidiary Colors in Commercial Food Blue No
February 1998 7 Original Structural Determination of Subsidiary Colors in Commercial Food Blue No. 1 (Brilliant Blue FCF) Product (Received September 1, 1997) Hirosh1 MATSUFUJI*1, Takashi KUSAKA*1, Masatoshi TSUKUDA*1, Makoto CHINO*1, Yoshiaki KATO*2, Mikio NAKAMURA*2, Yukihiro GODA*3, Masatake TOYODA*3 and Mitsuharu TAKEDA*1 (*1College of Bioresource Sciences, Nihon University: 3-34-1, Shimouma, Setagaya-ku, Tokyo 145-0002, Japan; *2San-Ei Gen F. F. I., Inc.: 1-1-11, Sanwa-cho, Toyonaka, Osaka 561-0828, Japan; *3National Institute of Health Sciences (NIHS): 1-18-1, Kamiyoga, Setagaya-ku, Tokyo 158-8501, Japan) HPLC analysis revealed that five subsidiary colors were present in a commercial Food Blue No. l (Brilliant Blue FCF) product. Among them, major subsidiary colors C, D, and E were isolated. On the bases of spectroscopic analyses, their structures were identified as the disodium salt of 2-[[4-[N-ethyl-N-(3-sulfophenylmethyl)amino]phenyl][4-[N-ethyl-N-(4-sulfo- phenylmethyl)amino]phenyl]methylio]benzenesulfonic acid, the disodium salt of 2-[[4- [N-ethyl-N-(2-sulfophenylmethyl) amino]phenyl][4-[N-ethyl-N-(3-sulfophenylmethyl) amino]- phenyl]methylio]benzenesulfonic acid, and the sodium salt of 2-[[4-(N-ethylamino)phenyl][4- [N-ethyl-N-(3-sulfophenylmethyl)amino]phenyl]methylio]benzenesulfonic acid, respectively. Key words: Food Blue No. 1; Brilliant Blue FCF; FD & C Blue No. 1; subsidiary color; HPLC; coal-tar dye ally, it is the disodium salt of 2-[bis[4-[N-ethyl- Introduction N-(3-sulfophenylmethyl) amino] phenyl] meth- Twelve coal-tar dyes are presently permitted ylio]benzensulfonic acid [OSBA-(m-EBASA)(m- as food colors in Japan. -

Reactions of Benzene & Its Derivatives
Organic Lecture Series ReactionsReactions ofof BenzeneBenzene && ItsIts DerivativesDerivatives Chapter 22 1 Organic Lecture Series Reactions of Benzene The most characteristic reaction of aromatic compounds is substitution at a ring carbon: Halogenation: FeCl3 H + Cl2 Cl + HCl Chlorobenzene Nitration: H2 SO4 HNO+ HNO3 2 + H2 O Nitrobenzene 2 Organic Lecture Series Reactions of Benzene Sulfonation: H 2 SO4 HSO+ SO3 3 H Benzenesulfonic acid Alkylation: AlX3 H + RX R + HX An alkylbenzene Acylation: O O AlX H + RCX 3 CR + HX An acylbenzene 3 Organic Lecture Series Carbon-Carbon Bond Formations: R RCl AlCl3 Arenes Alkylbenzenes 4 Organic Lecture Series Electrophilic Aromatic Substitution • Electrophilic aromatic substitution: a reaction in which a hydrogen atom of an aromatic ring is replaced by an electrophile H E + + + E + H • In this section: – several common types of electrophiles – how each is generated – the mechanism by which each replaces hydrogen 5 Organic Lecture Series EAS: General Mechanism • A general mechanism slow, rate + determining H Step 1: H + E+ E El e ctro - Resonance-stabilized phile cation intermediate + H fast Step 2: E + H+ E • Key question: What is the electrophile and how is it generated? 6 Organic Lecture Series + + 7 Organic Lecture Series Chlorination Step 1: formation of a chloronium ion Cl Cl + + - - Cl Cl+ Fe Cl Cl Cl Fe Cl Cl Fe Cl4 Cl Cl Chlorine Ferric chloride A molecular complex An ion pair (a Lewis (a Lewis with a positive charge containing a base) acid) on ch lorine ch loronium ion Step 2: attack of -

Intermediate Chemicals for Dyes Appendix to the Tariff
Harmonized Tariff Schedule of the United States (2004) Annotated for Statistical Reporting Purposes INTERMEDIATE CHEMICALS FOR DYES APPENDIX Harmonized Tariff Schedule of the United States (2004) Annotated for Statistical Reporting Purposes INTERMEDIATE CHEMICALS FOR DYES APPENDIX 2 This is supposed to be a blank page Harmonized Tariff Schedule of the United States (2004) Annotated for Statistical Reporting Purposes INTERMEDIATE CHEMICALS FOR DYES APPENDIX 3 This appendix enumerates those intermediate chemicals for dyes which are eligible for duty-free treatment under the provisions of general note 14 of the tariff schedule. Product CAS Number Acetaldehyde, (1,3-dihydro-1,3,3-trimethyl-2H-indol-2-ylidene)- .............................................84-83-3 Acetamide, N-(3-amino-4-methoxyphenyl)- ...........................................................6375-47-9 Acetamide, N-(3-aminophenyl)- .....................................................................102-28-3 Acetamide, N-(4-aminophenyl)- .....................................................................122-80-5 Acetamide, N-(3-aminophenyl)-, monohydrochloride .....................................................621-35-2 Acetamide, N-(4-aminophenyl)-N-methyl- .............................................................119-63-1 Acetamide, N-(2,5-dimethoxy phenyl)- ...............................................................3467-59-2 Acetamide, N-(7-hydroxy-1-naphthalenyl- ............................................................6470-18-4 Acetamide, N-(2-methoxy-5-methyl -

Snoop Safety Data Sheet, New Zealand (SDS-SNOOP-NZ
Snoop ® Safety Data Sheet According to the Hazardous Substances and New Organisms Act (1996) Date of Issue: 06/10/2019 Version: 1.0 SECTION 1: IDENTIFICATION OF THE SUBSTANCE OR MIXTURE AND OF THE SUPPLIER 1.1. Product Name Product Form: Mixture Product Name: Snoop ® 1.2. Other Names Not available 1.3. Recommended Use Snoop® is a proprietary blend of water, non-ionic surfactants, and a bacterialcide. 1.4. Company Name, Address And Contact Details Company Distributor Swagelok Manufacturing Company, LLC Enter your contact information here 29495 F.A. Lennon Drive Solon, Ohio 44139 440-519-4000 www.swagelok.com 1.5. Emergency Phone Number Emergency Number : INFOTRAC: (800) 535-5035 SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification Of The Substance Or Mixture GHS-NZ classification Not classified as a hazardous chemical. 2.2. GHS Label Elements, Including Precautionary Statements GHS-NZ Labeling No labelling applicable 2.3. Other hazards which do not result in classification Exposure may aggravate pre-existing eye, skin, or respiratory conditions. 2.4. Unknown Acute Toxicity (GHS-NZ) No data available SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTS 3.1. Substance Not applicable 3.2. Mixture Name Synonyms Product Identifier % * GHS Ingredient Classification Water AQUA / Aqua (CAS-No.) 7732-18-5 > 99.44 Not classified Benzenesulfonic acid, mono- Benzenesulfonic acid, mono- (CAS-No.) 68649-00-3 0.255 Not classified C9-17-branched alkyl C9-17-branched alkyl derivatives, compounds with derivatives, isopropylamine 2-propanamine / C9-17 salts Branched alkylbenzenesulfonic acid, isopropylamine salt / Benzenesulfonic acid, mono- branched alkyl(C9-17) derivatives, compounds with 2-propanamine Dodecylbenzenesulfonic Benzenesulfonic acid, (CAS-No.) 26264-05-1 0.207 9.3C: Ecotoxicity to terrestrial acid, isopropylamine salt dodecyl-, compound with 2- vertebrates C, H433 propanamine (1:1) / Dodecylbenzenesulfonic acid 6.1D: Acute Tox. -

Alkylsulfonic Acids, Liquid
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION Product Name : ALKYLSULFONIC ACIDS, LIQUID Other Names : (C10-16) ALKYLBENZENESULFONIC ACID BENZENESULFONIC ACID, C10-16 ALKYL DERIVATIVES Uses : Feedstock for detergent derivative manufacture. Organisation Location Telephone Ask For 2 Swettenham Road Minto NSW 2566 02- Technical Redox Pty Ltd Australia 97333000 Officer 131126 Poisons Information Westmead NSW 1800- Centre 251525 2. HAZARD IDENTIFICATION Hazardous according to criteria of NOHSC CORROSIVE Risk Phrases R35 Causes severe burns. R22 Harmful if swallowed. Safety Phrases In case of contact with eyes, rinse immediately with plenty of water and seek S26 medical advice. S28:DOBENZ After contact with skin, wash immediately with plenty of water. S36/37/39 Wear suitable protective clothing, gloves and eye/face protection. ERMA New Zealand Approval Code : No Data HSNO Hazard Classification : No Data This Material Safety Data Sheet may not provide exhaustive guidance for all HSNO Controls assigned to this substance. The ERMA website www.ermanz.govt.nz should be consulted for a full list of triggered controls and cited regulations 3. COMPOSITION/INFORMATION ON INGREDIENTS Chemical Entity CAS No. Proportions (%) LINEAR ALKYL BENZENE SULPHONIC ACID [68584-22-5] 100 4. FIRST AID MEASURES Description of necessary measures according to routes of exposure Swallowed If swallowed, do NOT induce vomiting. Transport to nearest medical facility for treatment. If vomiting occurs naturally, keep head below hips to prevent aspiration. Eye Immediately flush eyes with large amounts of water holding eyelids open. Transport to the nearest medical facility for treatment. Skin Remove contaminated clothing. Immediately flush skin with plenty of water. Transport to the nearest medical facility for treatment. -
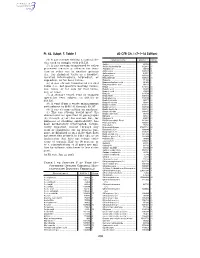
40 CFR Ch. I (7–1–14 Edition) Pt. 63, Subpt. F, Table 1
Pt. 63, Subpt. F, Table 1 40 CFR Ch. I (7–1–14 Edition) (4) A gas stream exiting a control de- Chemical name a CAS No. b Group vice used to comply with § 63.113. Aniline ................................................ 62533 I (5) A gas stream transferred to other Aniline hydrochloride ......................... 142041 III processes (on-site or off-site) for reac- Anisidine (o-) ..................................... 90040 II tion or other use in another process Anthracene ........................................ 120127 V (i.e., for chemical value as a product, Anthraquinone ................................... 84651 III Azobenzene ....................................... 103333 I isolated intermediate, byproduct, or Benzaldehyde .................................... 100527 III coproduct, or for heat value). Benzene ............................................ 71432 I (6) A gas stream transferred for fuel Benzenedisulfonic acid ...................... 98486 I Benzenesulfonic acid ........................ 98113 I value (i.e., net positive heating value), Benzil ................................................. 134816 III use, reuse, or for sale for fuel value, Benzilic acid ...................................... 76937 III use, or reuse. Benzoic acid ...................................... 65850 III Benzoin .............................................. 119539 III (7) A storage vessel vent or transfer Benzonitrile ........................................ 100470 III operation vent subject to § 63.119 or Benzophenone ................................. -

Aromatic Electrophilic Substitution: The Arenium Ion Mechanism
2/15/2020 ELECTROPHILIC AROMATIC SUBSTITUTION 1 Aromatic Electrophilic substitution: The arenium ion mechanism. Orientation and reactivity, energy profile diagram. The ortho / para ratio, ipso attack, orientation in other ring system, quantitative treatment of reactivity in substrates and electrophiles. Diazonium coupling Vilsmeir reaction Gatterman-Koch reaction 2 1 2/15/2020 ELECTROPHILIC AROMATIC SUBSTITUTION Both BENZENE and ALKENE are susceptible to E+ attack due to their exposed electrons 3 ELECTROPHILIC ADDITION IN ALKENE H H Addition Reaction E Nu E E Nu H H WHY ELECTROPHILIC ATTACK IN BENZENE? Theory The high electron density of the ring makes it open to attack by electrophiles HOWEVER... Because the mechanism involves an initial disruption to the ring, electrophiles will have to be more powerful than those which react with alkenes. 4 2 2/15/2020 A fully delocalised ring is stable so will resist ELECTROPHILIC ADDITION. H H E Addition Reaction E Nu X E Nu H H STABLE DELOCALISED SYSTEM DOES NOT FORM THIS PRODUCT SINCE LESS STABLE THAN STARTING MATERIAL DUE TO LOSS OF AROMATICITY ELECTRONS ARE NOT DELOCALISED AROUND THE WHOLE RING - LESS STABLE THEREFORE, BENZENE UNDERGOES SUBSTITUTION REACTION RATHER THAN ADDITION REACTION 5 ARENIUM ION, ITS MECHANISM, SE2 REACTION The general equation for this reaction is: Generation of E+ Catalyst E Nu E + Nu STEP I Arenium ion, Wheland Slow or intermediate or RDS Complex 6 Carbocation, Sp3 Hybridized due to new bonded electrophile 3 2/15/2020 Although the Wheland intermediate is stabilized by resonance •we have clearly lost the aromatic stabilization of the starting material and hence the addition of the electrophile is going to be the slow step (rds = rate determining step). -

Linear Alkylbenzene Sulfonate (Las)
OECD SIDS LINEAR ALKYLBENZENE SULFONATE (LAS) FOREWORD INTRODUCITON LINEAR ALKYLBENZENE SULFONATE (LAS) 1322-98-1 Decylbenzene sulfonic acid, sodium salt 25155-30-0 Dodecylbenzene sulfonic acid, sodium salt 26248-24-8 Tridecylbenzene sulfonic acid, sodium salt 27636-75-5 Undecylbenzene sulfonic acid, sodium salt 68081-81-2 C10-16 Monoalkylbenzene sulfonic acid, sodium salt 68411-30-3 C10-13 Alkylbenzene sulfonic acid, sodium salt 69669-44-9 C10-14 Alkyl deriv benzene sulfonic acid, sodium salt 85117-50-6 C10-14 Monoalkylbenzene sulfonic acid, sodium salt 90194-45-9 C10-13 Alkyl deriv benzene sulfonic acid, sodium salt 127184-52-5 4-C 10-13-sec Alkyl deriv benzene sulfonic acid, sodium salt UNEP PUBLICATIONS 1 OECD SIDS LINEAR ALKYLBENZENE SULFONATE (LAS) SIDS INITIAL ASSESSMENT REPORT For 20th SIAM Paris, France, 19-21 April, 2005 1. Chemical Name: Linear Alkylbenzene Sulfonate (LAS) 2. CAS Numbers: 1322-98-1 Decylbenzene sulfonic acid, sodium salt 25155-30-0 Dodecylbenzene sulfonic acid, sodium salt 26248-24-8 Tridecylbenzene sulfonic acid, sodium salt 27636-75-5 Undecylbenzene sulfonic acid, sodium salt 68081-81-2 C10-16 Monoalkylbenzene sulfonic acid, sodium salt 68411-30-3 C10-13 Alkylbenzene sulfonic acid, sodium salt 69669-44-9 C10-14 Alkyl deriv benzene sulfonic acid, sodium salt 85117-50-6 C10-14 Monoalkylbenzene sulfonic acid, sodium salt 90194-45-9 C10-13 Alkyl deriv benzene sulfonic acid, sodium salt 127184-52-5 4-C10-13-sec Alkyl deriv benzene sulfonic acid, sodium salt 3. Sponsor Country: United States National SIDS Contact Point in Sponsor Country: Oscar Hernandez, Director U.S. -

Aldrich Phosphorus and Sulfur Compounds
Aldrich Phosphorus and Sulfur Compounds Library Listing – 822 spectra Subset of Aldrich FT-IR Library related aldehydes and ketones. The Aldrich Material-Specific FT-IR Library collection represents a wide variety of the Aldrich Handbook of Fine Chemicals' most common chemicals divided by similar functional groups. These spectra were assembled from the Aldrich Collections of FT-IR Spectra Editions I or II, and the data has been carefully examined and processed by Thermo Fisher Scientific. Aldrich Phosphorus and Sulfur Compounds Index Compound Name Index Compound Name 245 ((1R)-(ENDO,ANTI))-(+)-3- 651 (2S)-(+)-GLYCIDYL 3- BROMOCAMPHOR-8- SULFONIC NITROBENZENESULFONATE,99% ACID, AMMONIUM SALT 649 (2S)-(+)-GLYCIDYL TOSYLATE, 246 ((1S)-(ENDO,ANTI))-(-)-3- 99% BROMOCAMPHOR-8- SULFONIC 352 (3,4-TOLUENEDITHIOLATO(2- ACID, AMMONIUM SALT ))ZINC HYDRATE 292 (+)-10- 402 (3- DICYCLOHEXYLSULFAMOYL-L- CHLOROPROPYL)DIPHENYLSULF ISOBORNEOL, 98% ONIUM TETRAFLUORO- BORATE, 242 (+/-)-10-CAMPHORSULFONIC ACID 97% MONOHYDRATE, 98% 295 (7R)-10,10-DIMETHYL-5-THIA-4- 243 (+/-)-10-CAMPHORSULFONIC ACID, AZATRICYCLO- (5.2.1.03,7)DEC-3- SODIUM SALT, 97% ENE-5,5-D 778 (-)-1-CHLORO-3-TOSYLAMIDO-7- 296 (7S)-10,10-DIMETHYL-5-THIA-4- AMINO-2- HEPTANONE AZATRICYCLO- (5.2.1.03,7)DEC-3- HYDROCHLORIDE, 98% ENE-5,5-D 291 (-)-10- 488 (PHENYLSULFINYL)(PHENYLSULF DICYCLOHEXYLSULFAMOYL-D- ONYL)METHANE, 95% ISOBORNEOL, 98% 439 (PHENYLSULFONYL)ACETONITRI 629 (-)-2,3-BUTANEDIOL DI-P- LE, 98% TOSYLATE, 99% 417 (R)-(+)-METHYL P-TOLYL 306 (-)-SINIGRIN MONOHYDRATE, 98% SULFOXIDE, 99% -

RHODACAL DS 4 SECTION 1: Identification of the Substance
SAFETY DATA SHEET RHODACAL DS 4 Revision Date 11/01/2018 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier - Trade name RHODACAL DS 4 1.2 Relevant identified uses of the substance or mixture and uses advised against Uses of the Substance / Mixture - Surfactants for various applications 1.3 Details of the supplier of the safety data sheet Company Solvay USA Inc., NOVECARE 504 Carnegie Center Princeton, NJ, 08540, US Telephone Number: 800-973-7873 1.4 Emergency telephone FOR EMERGENCIES INVOLVING A SPILL, LEAK, FIRE, EXPOSURE OR ACCIDENT, CONTACT CHEMTREC (24-Hour Number): 800-424-9300 within the United States and Canada, or 703-527-3887 for international collect calls. SECTION 2: Hazards identification Although OSHA has not adopted the environmental portion of the GHS regulations, this document may include information on environmental effects. 2.1 Classification of the substance or mixture HCS 2012 (29 CFR 1910.1200) Skin irritation, Category 2 H315: Causes skin irritation. Serious eye damage, Category 1 H318: Causes serious eye damage. 2.2 Label elements HCS 2012 (29 CFR 1910.1200) Pictogram Signal Word - Danger Hazard Statements - H315 Causes skin irritation. - H318 Causes serious eye damage. Precautionary Statements Prevention PRCO90002067 Version : 1.04 / US ( Z8 ) www.solvay.com 1 / 18 SAFETY DATA SHEET RHODACAL DS 4 Revision Date 11/01/2018 - P264 Wash skin thoroughly after handling. - P280 Wear protective gloves/ eye protection/ face protection. Response - P302 + P352 IF ON SKIN: Wash with plenty of soap and water. - P305 + P351 + P338 + P310 IF IN EYES: Rinse cautiously with water for several minutes. -

+ E X (+ H+ X–) Slow Fast — Step — E Regenerates Disrupts Aromatic X Aromatic Cycle Cycle H H H E E E
Lecture outline Ch 17 — Electrophilic Aromatic Substitution (EAS) and Nucleophilic Aromatic Substitution Electrophilic aromatic substitution (EAS) involves replacement of an H by an electrophile... (complete the resonance structures below, and add curvy arrows) H E + E X (+ H+ X–) slow fast — step — E regenerates disrupts aromatic X aromatic cycle cycle H H H E E E complete the resonance structures actual arenium ion intermediate: The positive charge must always be delocalized to what ring positions relative to the site of E+ attack? Recall that in many reactions of alkenes, initial addition of an electrophile (e.g. H+ or "Br+") is followed by addition of a nucleophile to the carbocation intermediate. Draw the product of addition below. Why is this pathway unfavorable in this case? H E X Specific EAS reactions — 1. Halogenation — X FeX3 + X2 (+ HX) (catalyst) (X = Cl, Br) (AlX3 also works as a catalyst) mechanism of formation of the "X+" electrophile: (add the lone pairs and charges) Br Br + FeBr3 Br Br FeBr3 Br Br FeBr3 (Fe3+ is e–-deficient, actual: ! a Lewis acid) EAS mechanism: (complete the resonance structures, and add curved arrows) H Br Br Br FeBr3 Br FeBr3 H H H Br Br Br As mentioned in the text, when the ring is strongly activated by substituents halogenation can take place without catalyst present. X2 alone is electrophilic enough. 2. Nitration — NO H2SO4 2 + HNO3 Formation of the electrophile starts with an acid-base reaction between sulfuric acid and nitric "acid". In this context we should probably call it "nitric base" O O O – H O O H 2 HO S O + HO N HO SO3 + H2O N N O O O O EAS mech: (fill in the missing bits) O H O N O – HSO4 N H or H O O 2 nitrobenzene etc (draw the resonance structures!) 3.