Advances in Bone Graft Substitutes in Spinal Fusion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Understanding Icd-10-Cm and Icd-10-Pcs 3Rd Edition Download Free
UNDERSTANDING ICD-10-CM AND ICD-10-PCS 3RD EDITION DOWNLOAD FREE Mary Jo Bowie | 9781305446410 | | | | | International Classification of Diseases, (ICD-10-CM/PCS) Transition - Background Palmer B. Manual placenta removal. A: Understanding ICD-10-CM and ICD-10-PCS 3rd edition International Classification of Diseases ICD is a common framework and language to report, compile, use and compare health information. Psychoanalysis Adlerian therapy Analytical therapy Mentalization-based treatment Transference focused psychotherapy. Hysteroscopy Vacuum aspiration. Every code begins with an alpha character, which is indicative of the chapter to which the code is classified. Search Compliance Understanding BC, resilience standards and how to comply Follow these nine steps to first identify relevant business continuity and resilience standards and, second, launch a successful While many coders use ICD lookup software to help them, referring to an ICD code book is invaluable to build an understanding of the classification system. Pregnancy test Leopold's maneuvers Prenatal testing. Endoscopy : Colonoscopy Anoscopy Capsule endoscopy Enteroscopy Proctoscopy Sigmoidoscopy Abdominal ultrasonography Defecography Double-contrast barium enema Endoanal ultrasound Enteroclysis Lower gastrointestinal series Small-bowel follow-through Transrectal ultrasonography Virtual colonoscopy. Psychosurgery Lobotomy Bilateral cingulotomy Multiple subpial transection Hemispherectomy Corpus callosotomy Anterior temporal lobectomy. While codes in sections are structured similarly to the Medical and Surgical section, there are a few exceptions. Send Feedback Do you have Understanding ICD-10-CM and ICD-10-PCS 3rd edition on the new website? Help Learn to edit Community portal Recent changes Upload file. D Radiation oncology. Stem cell transplantation Hematopoietic stem cell transplantation. The primary distinctions are:. Palmer Joseph C. -

2011 Abstracts 06�28�11 Sm
MID-AMERICA ORTHOPAEDIC ASSOCIATION 29 th Annual Meeting April 6-10, 2011 Hilton Tucson El Conquistador Resort Tucson, AZ NOTE: Disclosure information is listed at the end of this document. MAOA FIRST PLENARY SESSION April 7, 2011 1. Peripheral Nerve Blocks and Incidence of Postoperative Neurogenic Complaints and Pain Scores *Randy R. Clark, M.D. Iowa City, IA John P. Albright, M.D. Iowa City, IA Richard C. Johnston, M.D. Iowa City, IA Peripheral nerve blocks (PNBs) are a common adjuvant for anesthesia. In our experience PNBs cause a significant incidence of severe pain and neurologic complaints. We instituted a previously validated questionnaire completed by patients at their first postoperative visit. We asked patients to indicate if they received a PNB and to rate their pain on a standardized pain scale at several points in the postoperative period. Patients indicated if they experienced severe pain, had to return to the ER, and if they experienced lasting neurologic complaints. Comparative data was collected on patients who received a PNB and those who did not receive a PNB (control). 307 patients completed the survey, 244 patients with PNBs and 63 control patients. There was a 39.8% incidence of neurologic complaints in patients who received PNBs as compared to 9.5% incidence in patients who did not receive a PNB, P < 0.001. There was 27.9% (PNB) versus 14.3% (control) incidence of severe pain, P 0.027. Twenty-four patients that received PNBs versus five control patients visited the ER, P 0.65. Patients who received PNBs had significantly better pain control immediately after surgery (P 0.02) and trended towards improved pain control the same night (P 0.055), but there was no difference in pain control the morning after surgery, 24 hours after surgery, and at the one week postoperative period (P 0.99, 0.19, and 0.88). -
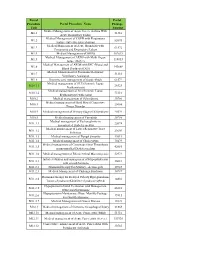
Portal Procedure Code Portal Procedure Name Portal Package Amount M1.1 Medical Management of Acute Severe Asthma with Acute
Portal Portal Procedure Portal Procedure Name Package Code Amount Medical Management of Acute Severe Asthma With M1.1 51310 Acute Respiratory Failure Medical Management of COPD with Respiratory M1.2 82095 Failure (Infective Exacerbation) Medical Management of Acute Bronchitis with M1.3 61572 Pneumonia and Respiratory Failure M1.4 Medical Management of ARDS 102619 Medical Management of ARDS with Multi Organ M1.5 118013 failure (R65.1) Medical Management of ARDS with DIC (Blood and M1.6 143668 Blood Products) (D65) Medical Management of Poisioning Requiring M1.7 51310 Ventilatory Assistance M1.8 Intensive care management of Septic Shock 61572 Medical management of SLE (Systemic Lupus M10.1.1 26323 Erythematosis) Medical management of Sle (Systemic Lupus M10.1.2 73116 Erythematosis) with sepsis M10.2 Medical management of Scleroderma 30786 Medical management of Mctd Mixed Connective M10.3 25654 Tissue Disorder M10.4 Medical management of Primary Sjogren\'S Syndrome 20524 M10.5 Medical management of Vasculitis 30786 Medical management of Pyelonephritis in M11.1.1 22074 uncontrolled Diabetes melitus Medical management of Lower Respiratoy Tract M11.1.2 23603 Infection M11.1.3 Medical management of Fungal Sinusitis 42013 M11.1.4 Medical management of Cholecystitis 30479 Medical management of Cavernous Sinus Thrombosis M11.1.5 42085 in uncontrolled Diabetes melitus M11.1.6 Medical management of Rhinocerebral Mucormycosis 52973 Initial evaluation and management of Hypopituitarism M11.2.1 26681 with growth harmone M11.2.2 Hormonal therapy for Pituitary -

Reconstructive Techniques in Musculoskeletal Tumor Surgery
Management of Pelvic and Extremity Bone Tumors Management of Pelvic Surgery Tumor in Musculoskeletal Techniques Reconstructive Reconstructive Techniques in Musculoskeletal Tumor Surgery Management of Pelvic and Extremity Bone Tumors Michaël P.A. Bus Michaël P.A. Michaël P.A. Bus 49073 Michaël Bus cover en kaartje.indd 1 28-02-18 11:33 Reconstructive Techniques in Musculoskeletal Tumor Surgery - Management of Pelvic and Extremity Bone Tumors Michaël P.A. Bus 49073 Michaël Bus.indd 1 21-02-18 09:08 Reconstructive Techniques in Musculoskeletal Tumor Surgery – Management of Pelvic and Extremity Bone Tumors PhD thesis, Leiden University, Leiden, the Netherlands Copyright © 2018 M.P.A. Bus, Amsterdam, the Netherlands All rights reserved. No parts of this thesis may be reproduced, stored in a retrieval system of any nature or by any means, without prior written consent of the author. The copyright of the articles that have been published has been transferred to the respective journals. ISBN/EAN 978-94-6332-316-1 Cover design Jeroen Luijt Photography (jeroenluijt.nl), Amsterdam, the Netherlands Lay-out Ferdinand van Nispen tot Pannerden, Citroenvlinder DTP & Vormgeving, my-thesis.nl Printing GVO Drukkers & Vormgevers B.V., Ede, the Netherlands The research projects in this thesis were supported by an unconditional research grant from implantcast GmbH, Buxtehude, Germany. Publication of this thesis was kindly supported by: Nederlandse Orthopaedische Vereniging (NOV), Universiteit Leiden, implantcast Benelux, Bislife Foundation, ChipSoft and Anna Fonds|NOREF. 49073 Michaël Bus.indd 2 21-02-18 09:08 Reconstructive Techniques in Musculoskeletal Tumor Surgery Management of Pelvic and Extremity Bone Tumors Proefschrift ter verkrijging van de graad van Doctor aan de Universiteit Leiden op gezag van Rector Magnificus prof. -

View Full Issue
The University of Pennsylvania Orthopaedic Journal Volume 31 June 2021 The University of Pennsylvania Orthopaedic Journal Volume 31, June 2021 Editorial Board Editors-in-Chief Sachin Gupta, MD Matthew Stein MD Faculty Advisors Samir Mehta, MD Section Editors Aymen Alqazzaz, MD Lauren Boden, MD Ashleigh Bush, MD Kathleen Collins, MD Ryan Deangelis, MD Bijan Delghani, MD George Fryhofer, MD Cody Hansen, MD Viviana Serra Lopez, MD Kendall Masada MD Brian Velasco MD Dainn Woo, MD Kelsey Young, MD VOLUME 31, JUNE 2021 iii The University of Pennsylvania Orthopaedic Journal Volume 31, June 2021 Table of Contents Introduction Section Letter from the Editors 1 Sachin Gupta, MD and Matthew Stein, MD Letter from the Chair 2 L. Scott Levin, MD, FACS Letter from the Program Director 5 Daniel C. Farber, MD Letter from the Vice Chair of Inclusion, Diversity and Equity 7 Lawrence Wells, MD Dedication: Dr. Craig Israelite, MD 8 Sachin Gupta, MD Faculty, Department, and Resident Updates Chief’s Corner: Academic Chief Update 9 Michael Eby, MD, Christina Nypaver, MD, and William Ryan, MD Class of 2012 Alumni Residents—Where are they now? 10 Matthew Stein, MD Dr. Kristy Weber Presidential Address and Q&A 12 Ashleigh N. Bush, MD Penn Center for Advanced Cartilage Repair and Osteochondritis Dissecans Treatment Update 16 Hannah M. Zlotnick, BS, Tomasina M. Leska, BS, Divya Talwar, PhD, MPH, Jay M. Patel, PhD, Jonathan H. Galarraga, BS, Jason A. Burdick, PhD, Theodore J. Ganley, MD, Robert L. Mauck, PhD, and James L. Carey, MD, MPH Medical Education in a COVID Era: The Role of Virtual Conference 19 Matthew Stein, MD, Samir Mehta, MD, L Scott Levin, MD, and Derek J. -

Fibular Autograft Dengan Cancellous Screw Pada Fraktur Neck Femur
LAPORAN KASUS FIBULAR AUTOGRAFT DENGAN CANCELLOUS SCREW PADA FRAKTUR NECK FEMUR Oleh dr. Made Bramantya Karna, Sp.OT (K) PROGRAM PENDIDIKAN DOKTER SPESIALIS PROGRAM STUDI SPESIALIS BEDAH ORTHOPAEDI DAN TRAUMATOLOGI UNIVERSITAS UDAYANA DENPASAR 2018 Laporan Kasus IDENTITAS Nama : IKS Jenis Kelamin : Laki-laki Umur : 40 tahun CM : 17001959 Alamat : Sidemen, Karangasem MRS : 13/01/17 ANAMNESIS Pasien datang sadar mengeluhkan sakit pinggul kanannya setelah jatuh 5 jam sebelum MRS. Pasien mengendarai sepeda motor, tiba-tiba kehilangan keseimbangannya kemudian terjatuh dan pinggul kanannya terbentur tanah. Riwayat tidak sadar (-), mual (-), muntah (-). Pasien tersebut dirujuk oleh Ahli Bedah Ortopedi dari Rumah Sakit Badung dengan CF Right Neck Femur. PEMERIKSAAN FISIK Regio Hip Kanan L: Bengkak (-), memar (-), deformitas (+) pemendekan dan rotasi eksternal, daya dipasang skin traction. F: Nyeri tekan (+), nadi a. Dorsalis pedis (+), CRT <2 ", sensasi normal M: Active ROM Hip terbatas karena rasa sakit Active ROM Genu terbatas karena rasa sakit Active ROM Ankle 30/40 Active ROM MTP-IP 0/90 1 PEMERIKSAAN PENUNJANG Foto Pelvis AP (RSUD Badung) Foto Femur Dextra AP/Lateral (RSUD Badung) DIAGNOSIS CF Right Neck Femur Garden Type II PENATALAKSANAAN Analgetik Imobilisasi dengan Skin Traction 5 kg P/ ORIF Screwing cannulated + Strut graft 2 Right Hip X-Ray AP View Post ORIF Screwing + Strut Graft Right Thigh X-Ray AP/Lateral View Post ORIF Screwing + Stut Graft 3 Clinical Picture Post ORIF Screwing + Strut Graft Clinical Picture Post ORIF Screwing + Strut Graft Follow up Foto Pelvis AP (12-9-2017) 4 DISKUSI KASUS Pasien laki-laki usia 40 tahun rujukan dari Ahli Bedah Ortopedi dari Rumah Sakit Badung dengan CF Right Neck Femur, datang sadar mengeluhkan sakit pinggul kanannya setelah jatuh 5 jam sebelum MRS. -

2013 Abstracts Revised 06�10�13 Sm
MID-AMERICA ORTHOPAEDIC ASSOCIATION 31 st Annual Meeting April 17-21, 2013 Omni Amelia Island Resort Amelia Island, FL Podium and Poster Abstracts NOTE: Disclosure information is listed at the end of this document. MAOA FIRST PLENARY SESSION April 18, 2013 1. Long-Term Outcomes of Modified Eden-Lange Tendon Transfer for Symptomatic Trapezius Paralysis from Spinal Accessory Nerve Injury Eric R. Wagner, M.D. Rochester, MN *Basseem T. Elhassan, M.D. Rochester, MN PURPOSE: The purpose of this study is to evaluate the outcome of multiple tendon transfers to the scapula to stabilize the scapulothoracic articulation in the treatment of symptomatic trapezius paralysis. METHODS: Thirteen patients, with average age of 25 years, had a history of trapezius paralysis secondary to spinal accessory nerve injury that failed to recover spontaneously or after nerve repair. The indications for surgery included shoulder pain and weakness and limited range of motion of the shoulder, specifically shoulder abduction. All patients underwent triple tendon transfer, including transfer of the levator scapulae with its bony insertion to the lateral aspect of the spine of the scapula, rhomboid minor with its bony insertion to the spine of the scapula just medial to the levator scapulae insertion, and rhomboid major tendinous insertion to the medial spine of the scapula and superomedial aspect of the infraspinatus fossa. All patients had a CT scan and ultrasound done at brace removal and beyond one year. RESULTS: At an average follow-up of 25 months (15-35), all patients had improvement of neck asymmetry, restoration of the scapula position compared to the opposite site, and no evidence of winging. -

Chapter 5 Arthroplasty for Proximal Humerus Fractures, Nonunions, and Malunions Edward W
Chapter 5 Arthroplasty for Proximal Humerus Fractures, Nonunions, and Malunions Edward W. Lee and Evan L. Flatow Treatment of complex fractures and fracture-dislocations of the proxi- mal humerus in both the acute and late settings represent some of the most difficult injuries to assess and treat in the shoulder girdle. While the vast majority of proximal humerus fractures (more than 85%) are nondisplaced and amenable to nonoperative treatment, the remaining minority may involve multiple fragments or parts, comminution, and articular surface damage and represent a significant therapeutic challenge. Based on Codman’s anatomical description of fractures about the proximal humerus [1], Neer [2] published a comprehensive four-part classification scheme of these complex injuries in 1970. Wide use of this system has allowed for more uniformity in evaluation and has directed treatment in this area for the last three decades. In conjunction with adequate evaluation of the proximal humeral anatomy, whether it is an acute fracture or the sequelae of an old injury, the therapeutic course should be guided by the surgical goal of anatomic restoration of the proximal humerus and patient-determined factors, such as age, function, and quality of remaining bone. With respect to acute fractures, all two-part, most three-part, and some four- part fractures are well treated by closed reduction or open reduction and internal fixation techniques. When soft bone, impaired head vas- cularity, or articular damage make fixation difficult or ill-advised, pros- thetic arthroplasty is a reasonable option. Use of a humeral head prosthesis for proximal humerus fractures was first reported in the 1950s. -

THE Bad Results of Open Reduction and Internal Fixation of Fractures Are Most
CHAPTER FIFTEEN FRACTURES OF THE SHAFT OF THE TIBIA HE bad results of open reduction and internal fixation of fractures are most commonly seen in fractures of the shaft of the tibia. There are several reasons Tfor this : the tibia is the commonest major long bone to be fractured; it is very commonly a compound fracture; it is a fracture which is easily exposed and therefore tempts inexperienced operators; it is a subcutaneous fracture and after plating is especially prone to defective wound healing accompanied by what in some quarters is euphemistically termed ' drainage/ We have still a long way to go before the best method of treating a fracture of the shaft of the tibia can be stated with finality. I feel sure that a closed method will eventually prevail, but we need mechanical aids to improve our control of the bone fragments. It is possible time will show that an intramedullary rod, introduced through the tibial tubercle, without exposing the fracture site, will be enough to enhance alignment as an adjuvant to closed methods. Used in this simple way the intramedullary rod will not be responsible for immobilisation; it will merely control alignment and prevent slipping of the reduced fracture. Most surgeons who practise internal fixation of tibial fractures do so with the idea that accurate coaptation of the fragments, combined with rigid fixation, enhances the ability of the fracture to unite. I have attempted to show in Chapter I that this mechanical approach to fracture healing is out of touch with biological reality. When viewing the excellent results which are often demonstrated as attributable to the plating of fractures of the tibia we must never forget that the best results of operative treatment are in cases which would also give excellent results by simple plaster fixation. -

How to Assess Shoulder Functionality: a Systematic Review of Existing Validated Outcome Measures
diagnostics Review How to Assess Shoulder Functionality: A Systematic Review of Existing Validated Outcome Measures Rocio Aldon-Villegas 1, Carmen Ridao-Fernández 1,* , Dolores Torres-Enamorado 2 and Gema Chamorro-Moriana 1 1 Research Group “Area of Physiotherapy” CTS-305, Department of Physiotherapy, University of Seville, 41009 Seville, Spain; [email protected] (R.A.-V.); [email protected] (G.C.-M.) 2 Research Group “Women, Well-being and Citizenship” SEJ066, Department of Nursing, University of Seville, 41930 Bormujos, Spain; [email protected] * Correspondence: [email protected] Abstract: The objective of this review was to compile validated functional shoulder assessment tools and analyse the methodological quality of their validations. Secondarily, we aimed to provide a comparison of the tools, including parameter descriptions, indications/applications, languages and operating instructions, to choose the most suitable for future clinical and research approaches. A systematic review (PRISMA) was conducted using: PubMed, WoS Scopus, CINHAL, Dialnet and reference lists until 2020. The main criteria for inclusion were that papers were original studies of vali- dated tools or validation studies. Pre-established tables showed tools, validations, items/components, etc. The QUADAS-2 and COSMIN-RB were used to assess the methodological quality of validations. Citation: Aldon-Villegas, R.; Ultimately, 85 studies were selected, 32 tools and 111 validations. Risk of bias scored lower than Ridao-Fernández, C.; applicability, and patient selection got the best scores (QUADAS-2). Internal consistency had the Torres-Enamorado, D.; highest quality and PROMs development the lowest (COSMIN-RB). Responsiveness was the most Chamorro-Moriana, G. How to analysed metric property. -
2012 Abstracts.Pdf 1.37 MB
25th EMSOS Annual Meeng CONTENT SESSION PELVIC TUMORS 1 SESSION CHONDROSARCOMA 10 SESSION FREE PAPER 1 - ONCOLOGY 17 SESSION NEW DRUGS/STRATEGIE 29 SESSION FREE PAPERS 2 - SURGERY 35 SESSION RADIOTHERAPY 47 SESSION SARCOMAGENESIS 53 SESSION FREE PAPER 3 - RARE TUMORS 57 SESSION FREE PAPER 4 - DIAGNOSIS 65 13th Symposium EMSOS Nurse Group NURSES AND ALLIED PROFESSIONS: ROLE IN CLINICAL RESEARCH 74 PROCEDURE IN SARCOMA PATIENTS 75 RESEARCH IN SARCOMA PATIENTS 78 PAIN IN SARCOMA PATIENTS 82 QUALITY OF LIFE IN SARCOMA PATIENTS 85 POSTER CHONDROSARCOMA 90 NEW DRUGS/STRATEGIES 110 PELVIC TUMORS 132 SARCOMAGENESIS 146 RADIOTHERAPY 150 ONCOLOGY 157 DIAGNOSIS 191 RARE TUMORS 221 SURGERY 249 NURSE GROUP 293 I Page Page Aach M. 137 Badalamen G. 22 Abate M. 20 Bahk W. J. 145, 291 Abdikarimov K. G. 166, 200, 209, 261, Bakhshai Y. 280 262, 263 Baldi G. G. 22 Abdurasulov R. R. 209 Balke M. 115 Abramo A. 276 Ballcels S. 64 Abudu A. 36, 72, 99, 118, Ban B. 54 AUTHORS INDEX AUTHORS 151, 167, 189, 232, Bandiera S. 23 249, 270, 277, 292 Banse X. 252 Abudu S. 237 Barban Brodano G. 23, 220 Adam V. 113 Barbier O. 110 Ae K. 27, 264 Barbieri E. 20 Afonso M. 122, 152 Barbieri M 86 Aggeliki Kalogeridi M. 153 Barnes D. J. 32 Ahrens H. 137, 285 Barry J. 169 Aido R. 225, 226 Bartoli M. S. 36, 102, 214, 237, Aigner R. 196 278 Alberghini M. 9, 15, 20, 29, 61, Basbozkurt M. 233, 234, 235, 289 63, 101, 129 Bastoni S. 1, 102 Alberni U. -

April 22, 2010
MID-AMERICA ORTHOPAEDIC ASSOCIATION 28 th Annual Meeting April 21-25, 2010 Hyatt Regency Lost Pines Resort and Spa Lost Pines (Austin), Texas NOTE: Disclosure information is listed at the end of this document. ------------------------------------------------------------------------------------------------------------------------------- MAOA FIRST PLENARY SESSION April 22, 2010 1. Patient Perceptions of Surgeon-Industry Relations Scott C. McGovern, M.D. Salisbury, MD *Robert T. Trousdale, M.D. Rochester, MN Bradford L. Currier, M.D. Rochester, MN INTRODUCTION: Little information is available on patients’ perceptions of physician-industry relationships identifying any differing views between surgical and nonsurgical patients. METHODS: Two groups of surgical patients representing primary total joint arthroplasty and instrumented spinal surgery were randomly selected from all primary THAs and TKAs or instrumented spinal fusions performed between 2003 and 2007. These were matched with cohorts of nonsurgical patients with correlating diagnoses. A comprehensive survey was sent; mailing response rate was 45%. We obtained 404 usable survey responses for matched cohorts. RESULTS: Survey responders were a mean age of 66 years and 51% were males. 93% agree or strongly agree that it is beneficial for doctors to advise the manufacturers of medical devices. 92% trust their doctor to do what is best for the patient. 82% agree that doctors should receive payment for designing implants, but 40% believe doctors should receive financial reimbursement for an advisory role. 71% agree that doctors should disclose a financial or advisory relationship. Only 27% agree that surgeon choice of implant is influenced by financial reimbursement. Nonsurgical groups respond more strongly that surgeon industry relationships increase the cost of medical care. Surgical patients were more inclined to allow doctors to make the sole choice of implants and were significantly less in favor of the patient choosing the implant.