Reconstructive Techniques in Musculoskeletal Tumor Surgery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Curriculum Vitae
CURRICULUM VITAE STEVEN GITELIS, M.D. PERSONAL DATA: Professional Address: Midwest Orthopaedics at Rush 1611 West Harrison St. Suite 300 Chicago, Illinois 60612 (312) 563-2600 E-Mail: [email protected] CURRENT APPOINTMENTS/ACTIVITIES: Trustee Rush University Associate Dean Surgery Rush Medical College Associate Chief Medical Officer Rush University Chief of Surgery Rush University President Elect Medical Staff Rush University Director Cancer Research Rush University Surgical Services Executive Committee Rush University Executive Committee Rush University Patient Safety Officer Orthopedic Surgery Rush University Rush Credentialing Committee Rush Medical Quality Committee Rush Surgical Quality Committee Rush Bylaws Committee Rush University Committee on Committees Ends 2012 Editor Emeritus Rush Orthopaedic Journal Vice Chairman Department of Orthopedic Surgery Rush Medical College Treasurer Medical Staff Rush University Medical Center Ends 2010 Rush University Committee on Committees Rush University Psychiatry Chairman Search Committee 2010 Past President Rush Surgical Society Treasurer 20th Century Orthopedic Association Biological Implants Committee AAOS Ends 2013 Chairman Task Force Technology Oversight Committee AAOS Ends 2010 Director Rush Center for Limb Preservation Rush Medical College Professor of Orthopaedic Oncology (Endowed Chair) Past President, Musculoskeletal Tumor Society Director, Section of Orthopedic Oncology Rush-Presbyterian-St. Luke's Medical Center, Chicago, Illinois Director, Bone and Tissue Bank of Gift of Hope -

Understanding Icd-10-Cm and Icd-10-Pcs 3Rd Edition Download Free
UNDERSTANDING ICD-10-CM AND ICD-10-PCS 3RD EDITION DOWNLOAD FREE Mary Jo Bowie | 9781305446410 | | | | | International Classification of Diseases, (ICD-10-CM/PCS) Transition - Background Palmer B. Manual placenta removal. A: Understanding ICD-10-CM and ICD-10-PCS 3rd edition International Classification of Diseases ICD is a common framework and language to report, compile, use and compare health information. Psychoanalysis Adlerian therapy Analytical therapy Mentalization-based treatment Transference focused psychotherapy. Hysteroscopy Vacuum aspiration. Every code begins with an alpha character, which is indicative of the chapter to which the code is classified. Search Compliance Understanding BC, resilience standards and how to comply Follow these nine steps to first identify relevant business continuity and resilience standards and, second, launch a successful While many coders use ICD lookup software to help them, referring to an ICD code book is invaluable to build an understanding of the classification system. Pregnancy test Leopold's maneuvers Prenatal testing. Endoscopy : Colonoscopy Anoscopy Capsule endoscopy Enteroscopy Proctoscopy Sigmoidoscopy Abdominal ultrasonography Defecography Double-contrast barium enema Endoanal ultrasound Enteroclysis Lower gastrointestinal series Small-bowel follow-through Transrectal ultrasonography Virtual colonoscopy. Psychosurgery Lobotomy Bilateral cingulotomy Multiple subpial transection Hemispherectomy Corpus callosotomy Anterior temporal lobectomy. While codes in sections are structured similarly to the Medical and Surgical section, there are a few exceptions. Send Feedback Do you have Understanding ICD-10-CM and ICD-10-PCS 3rd edition on the new website? Help Learn to edit Community portal Recent changes Upload file. D Radiation oncology. Stem cell transplantation Hematopoietic stem cell transplantation. The primary distinctions are:. Palmer Joseph C. -

Sarcoma of the Lower Limb: Reconstructive Surgeon's Perspective
Published online: 2019-05-08 THIEME Review Article 55 Sarcoma of the Lower Limb: Reconstructive Surgeon’s Perspective Zhixue Lim1 Sophia A. Strike1 Mark E. Puhaindran1 1Department of Hand and Reconstructive Microsurgery, National Address for correspondence Zhixue Lim, MRCS (Edin), Department of University Hospital, Singapore, Singapore Hand and Reconstructive Microsurgery, National University Hospital, NUHS Tower Block, Level 11, 1E Kent Ridge Road, Singapore 119228, Singapore (e-mail: [email protected]). Indian J Plast Surg 2019;52:55–61 Abstract Management of sarcomas in the lower extremities have evolved from amputations to limb-preserving surgeries with evidence to support that they have equal overall Keywords survival, albeit with better functional outcome. The challenge of reconstruction lies ► sarcoma in providing a durable, functional, and aesthetically pleasing limb. However, limb- ► lower extremity preserving intention should not delay interventions that provide a survival benefit such ► reconstruction as chemotherapy and radiotherapy. The advent of radiotherapy and chemotherapy also ► vascularised bone has implications on wound healing and should be considered during the reconstruc- graft tive process. This article reviews the methodical approach, reconstructive strategies, ► soft tissue and considerations for the reconstructive surgeon with respect to the lower extremity reconstruction after sarcoma excision. who described a systematic approach to limb-preserving Introduction surgery in his article “Conservative surgery in the treatment Sarcomas are a diverse group of neoplasms that account of bone tumours,” where he shared his personal experience for approximately 1% of adult malignancies and 7 to 15% of in combining radiotherapy and surgical excision, with the 1 pediatric malignancies. The range of histological subtypes possibility of bone transplantation after resection of bone contributes to the varied prognoses. -

Rotationplasty Offers Greater Functionality for Patients with Cancer
Johns Hopkins Framework Orthopaedic Surgery NEWS FROM JOHNS HOPKINS MEDICINE Winter 2016 Rotationplasty Offers Greater Functionality for Patients with Cancer aya oberstein was diagnosed with osteosarcoma of Mthe distal femur in 2012, at age 9. After she completed chemotherapy, Maya’s treatment options Since Maya Oberstein’s initial 9.5-hour procedure, Carol Morris has been following were an above-knee up every six months to monitor her progress. amputation, limb salvage side. Cosmetically, if you’re sitting, it’s nice if the with an internal prosthesis or a knees are even.” Morris recalls her initial reluctance about the more unconventional approach: Post-op procedure. “I thought it was a physically challenging If you believe in yourself, rotationplasty. Carol Morris, thing to do to a child when prosthetics had made chief of Johns Hopkins’ tremendous advancements,” she says. “As I gained you can do anything.” more experience in the field, I began to appreciate —MAYA OBERSTEIN Orthopaedic Oncology Division, the limitations of internal prostheses and the is one of a select group of surgeons functionality rotationplasty could provide. For the in the United States who perform right parents and the right child, under the right set of circumstances, rotationplasty is a good operation. this alternative reconstructive It’s much more functional than an above-knee procedure. Morris counseled Maya amputation.” Traditional prostheses, especially growing and her family on the available protheses, are more restrictive than the modified options. “She was the first doctor transtibial prosthesis, limiting a patient’s ability to who asked me how I was feeling,” participate not only in sports, but in typical activities such as running, dancing and jumping. -

Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers Version 4.0 – October 2013
Children’s OncologyLong-Term Group Long-Term Follow-UpFollow-Up Guidelines for Survivors of Childhood,Guidelines Adolescent, and Young Adult Cancer Version 4.0 – October 2013 for Survivors of Childhood, Adolescent, and Young Adult Cancers www.survivorshipguidelines.org Version 4.0 October 2013 Copyright 2013 © Children’s Oncology Group All rights reserved worldwide Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers Version 4.0 – October 2013 www.survivorshipguidelines.org Copyright 2013 © Children’s Oncology Group All rights reserved worldwide Contents Content Outline vi Section # Page Gender Potential Late Effect Abstract vii 13 15 Female Gonadal dysfunction (ovarian) Disclaimer and Notice of Proprietary Rights viii 14 17 Acute myeloid leukemia; myelodysplasia Guidelines Panel of Experts x 15 18 Pulmonary fibrosis Guidelines Task Force Membership 2009–2012 xi 16 19 Cataracts Guidelines Health Link Authors xvi 17 20 Urinary tract toxicity Guidelines Health Link Reviewers xviii 18 21 Bladder malignancy Guidelines Development Task Force – Initial Versions xix 19 22 Renal toxicity Guidelines Reviewers – Initial Versions xx 20 23 Ototoxicity Introductory Material xxii 21 25 Peripheral sensory neuropathy; Introduction xxiii 22 26 Renal toxicity Explanation of Scoring xxviii (n/a) [Removed from v4: Dyslipidemia] Instructions for Use xxix 23 27 Neurocognitive deficits New to Version 4.0 xxxiv 24 29 Clinical leukoencephalopathy 25 31 No known late effects 26 32 Hepatic dysfunction; veno-occlusive disease -

Statistical Analysis Plan
~ GILEAU STATISTICAL ANALYSIS PLAN Study Title: A Phase 2, Randomized, Open Label Study to Evaluate the Efficacy and Safety ofTenofovir Alafenamide (fAF) versus Tenofovir Disoproxil Fumarate (fDF)-containing Regimens in Subjects with Chmnic HBV Infection and Stage 2 or Greater Chronic Kidney Disease Who Have Received a Liver Transplant Name of Test Drug: Tenofovir Alafenamide (fAF) Study Number: GS-US-320-3912 Protocol Version (Date): Amendment 2 (28 March 2017) Analysis Type: Week 24 Analysis Plan Version: Version 1.0 Analysis Plan Date: 19 March 2018 ..._ ...... Analysis Plan Author(s): PD______ CONFIDENTIAL AND PROPRIETARY INFORMATION TAF GS-US-320-3912 Statistical Analysis Plan – Week 24 Analysis Version 1.0 TABLE OF CONTENTS TABLE OF CONTENTS ..............................................................................................................................................2 LIST OF IN-TEXT TABLES........................................................................................................................................4 LIST OF ABBREVIATIONS........................................................................................................................................5 1. INTRODUCTION ................................................................................................................................................8 1.1. Study Objectives ......................................................................................................................................8 1.2. Study Design ............................................................................................................................................9 -

Van Nes Rotationplasty As a Treatment Method for Ewing's
CASE REPORT – OPEN ACCESS International Journal of Surgery Case Reports 4 (2013) 893–897 Contents lists available at ScienceDirect International Journal of Surgery Case Reports jou rnal homepage: www.casereports.com Van Nes rotationplasty as a treatment method for Ewing’s sarcoma in ଝ a 14-month-old ∗ Jagmeet S. Bhamra , Hani B. Abdul-Jabar, David McKenna, Stephen Ng Man Sun, Elizabeth Gillott, Robin Pollock Bone Tumour Unit, Royal National Orthopaedic Hospital, Brockley Hill, Stanmore, Middlesex HA7 4LP, UK a r t i c l e i n f o a b s t r a c t Article history: INTRODUCTION: In recent years, the rotationplasty procedure has become popular amongst tumour sur- Received 11 January 2013 geons as an alternative to endoprosthetic replacement or amputation. There are very few documented Received in revised form 26 June 2013 cases of this technique in young patients with malignancy. Accepted 19 July 2013 PRESENTATION OF CASE: We describe an extremely rare case of Ewing’s sarcoma in a 14-month-old Available online 6 August 2013 boy that involved the entire length of the left femur. At initial presentation, pulmonary metastatic spread had occurred and there was no neurovascular involvement. Complete response to neo-adjuvant Keywords: chemotherapy was achieved prior to performing the definitive surgical procedure. 14-Month-old DISCUSSION: This case highlights the many reconstructive options and difficulties encountered in man- Ewing’s sarcoma Femur aging such extremely young patients with aggressive malignant disease. In this case, a complete femoral Outcome excision was necessary and various treatment options were explored. These included irradiation and Treatment options re-implantation, endoprosthetic replacement and manufacturing a custom growing prosthesis. -

The Endlock Tumor Prosthesis with Short-Length Fixation: a Clinical Study
An Original Study The Endlock Tumor Prosthesis With Short-Length Fixation: A Clinical Study Leonhard E. Ramseier, MD, Clement M. Werner, MD, Hilaire A. C. Jacob, PhD, and G. Ulrich Exner, MD early stability has been reported,10 and the advantages of ABSTRACT cementless fixation in revision cases have been maintained. Anchorage of segmental replacement prostheses in However, fixation over a long length of stem has the dis- diaphyseal bone remains a challenge in lower limb advantage of potential stress-shielding of the surrounding reconstructions. We developed and studied a new pros- bone.8,10-18 Interestingly, the prosthetic design is seldom thesis design that features an intramedullary anchorage implicated as a possible cause of aseptic loosening.10,19 system for which finite element analysis predicted favor- Encouraged by the good clinical and radiologic results able bone remodeling. We retrospectively analyzed the 20 cases of all patients who underwent implantation of the reported with the thrust plate hip prosthesis, a device new stem. Their data were prospectively collected. with a very short segmental fixation to bone, we proposed Twenty-four patients (25 prosthetic reconstructions a new design of diaphyseal anchorage and explored its using diaphyseal fixation of the prosthesis) had 18 pri- theoretical advantages by finite element analysis.21 The mary implantations and 7 revision cases. At a mean fol- short-length fixation in this design has shown a definitive low-up of 61 months, TESS (Toronto Extremity Salvage advantage over long-length fixation. The stress pattern Score) and MSTS (Musculoskeletal Tumor Society within the bone surrounding the prosthesis confirmed that Rating Scale score) were 80% and 65% that of a nor- shortening of the on-growth area in length increases the mal extremity, respectively. -

2011 Abstracts 06�28�11 Sm
MID-AMERICA ORTHOPAEDIC ASSOCIATION 29 th Annual Meeting April 6-10, 2011 Hilton Tucson El Conquistador Resort Tucson, AZ NOTE: Disclosure information is listed at the end of this document. MAOA FIRST PLENARY SESSION April 7, 2011 1. Peripheral Nerve Blocks and Incidence of Postoperative Neurogenic Complaints and Pain Scores *Randy R. Clark, M.D. Iowa City, IA John P. Albright, M.D. Iowa City, IA Richard C. Johnston, M.D. Iowa City, IA Peripheral nerve blocks (PNBs) are a common adjuvant for anesthesia. In our experience PNBs cause a significant incidence of severe pain and neurologic complaints. We instituted a previously validated questionnaire completed by patients at their first postoperative visit. We asked patients to indicate if they received a PNB and to rate their pain on a standardized pain scale at several points in the postoperative period. Patients indicated if they experienced severe pain, had to return to the ER, and if they experienced lasting neurologic complaints. Comparative data was collected on patients who received a PNB and those who did not receive a PNB (control). 307 patients completed the survey, 244 patients with PNBs and 63 control patients. There was a 39.8% incidence of neurologic complaints in patients who received PNBs as compared to 9.5% incidence in patients who did not receive a PNB, P < 0.001. There was 27.9% (PNB) versus 14.3% (control) incidence of severe pain, P 0.027. Twenty-four patients that received PNBs versus five control patients visited the ER, P 0.65. Patients who received PNBs had significantly better pain control immediately after surgery (P 0.02) and trended towards improved pain control the same night (P 0.055), but there was no difference in pain control the morning after surgery, 24 hours after surgery, and at the one week postoperative period (P 0.99, 0.19, and 0.88). -

Advances in Bone Graft Substitutes in Spinal Fusion
17 Advances in Bone Graft Substitutes in Spinal Fusion Michael N. Tzermiadianos, Alexander G. Hadjipavlou, and John N. Gaitanis University of Kriti Medical School Iraklio Kriti, Greece Bone grafting is essential for reconstruction of spinal defects and a prerequisite to obtaining solid arthrodesis imperative to spinal stability after reconstructive surgery [1]. Spinal fusion is commonly achieved by the adjunctive use of interbody or onlay cortical bone grafts (autograft or allograft). Success depends on factors such as the patient’s age, sufficiency of local blood supply, degrees of postoperative movement, and, importantly, the physical and biological charac- teristics of the graft matrix. Early attempts at bone grafting date back more than 500 years to the Arab, indigenous Peruvian, and Aztec cultures. In modern times, the first documented case of autogenous bone grafting was reported by Merem in 1810, and the first successful allografting case has been attributed to Macewn in 1881 [2]. Our present knowledge and scientific base for understanding the biology, banking, and widespread clinical applications of bone grafting is largely due to the work of Albee [3], Barth [4], Lexter [5], Phemister [6], and Seen [7] during the late nineteenth and early twentieth centu- ries. These substantive scientific contributions have made bone grafting techniques common and relatively effective clinical procedures. There are three biological processes that impact the success or failure of bone graft: osteogenesis, osteoconduction, and osteoinduction [8]. Osteogenesis refers to the process whereby bone forms directly from living cells, such as the stem cells within autogenous bone. Osteoconduction describes the process in which bone grows into and along the surface of a biocompatible structure when placed in direct apposition to host bone through the process of intramembranous bone formation. -

Dr. Cornett Named L. Thomas Hood, MD Professor of Orthopaedic Surgery
Breaking News for alumni and friends of the University of Nebraska Medical Center Department of Orthopaedic Surgery Nebraska Medicine—Bellevue locations. For his dedication to teaching, the orthopaedic residents selected Dr. Cornett to receive the department’s Outstanding Instructor Award. After completing a master’s degree in physical therapy, Dr. Cornett obtained his medical degree from UNMC. He completed residency at the University of Wisconsin in Madison and a spine surgery fellowship at the University of Pittsburgh Medical Center. Dr. Cornett’s clinical expertise is all aspects of adult spine surgery (cervical, thoracic and lumbar). In his spine-only practice, he treats a variety of conditions, including degenerative Dr. Cornett named L. Thomas Hood, MD conditions/arthritis, spinal stenosis, myelopathy, spondylolisthesis, disc Professor of Orthopaedic Surgery herniations, instability, scoliosis, tumors and trauma. Chris Cornett, MD, associate professor of Orthopaedic Surgery and Dr. Cornett serves on multiple UNMC Rehabilitation, has been named to the second L. Thomas Hood, MD, committees, including the Continuing Professor of Orthopaedic Surgery. Medical Education Committee and the This professorship is awarded to Dr. Cornett joined the Department of Faculty Senate. He also participates in faculty who have followed in Dr. Orthopaedic Surgery and Rehabilitation several Nebraska Medicine advisory Hood’s footsteps by establishing high in 2011 and is the department’s and operational committees. He is standards in patient care, education vice-chair of clinical services. He is an active member of many local and and research—inspiring orthopaedic also the medical director of both the national professional organizations surgeons of the future to maintain the Comprehensive Spine Program and and societies, including the American department’s mission and standard of Outpatient Physical & Occupational Academy of Orthopaedic Surgeons excellence. -
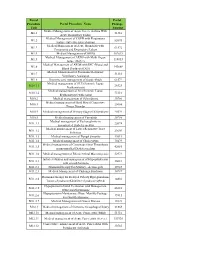
Portal Procedure Code Portal Procedure Name Portal Package Amount M1.1 Medical Management of Acute Severe Asthma with Acute
Portal Portal Procedure Portal Procedure Name Package Code Amount Medical Management of Acute Severe Asthma With M1.1 51310 Acute Respiratory Failure Medical Management of COPD with Respiratory M1.2 82095 Failure (Infective Exacerbation) Medical Management of Acute Bronchitis with M1.3 61572 Pneumonia and Respiratory Failure M1.4 Medical Management of ARDS 102619 Medical Management of ARDS with Multi Organ M1.5 118013 failure (R65.1) Medical Management of ARDS with DIC (Blood and M1.6 143668 Blood Products) (D65) Medical Management of Poisioning Requiring M1.7 51310 Ventilatory Assistance M1.8 Intensive care management of Septic Shock 61572 Medical management of SLE (Systemic Lupus M10.1.1 26323 Erythematosis) Medical management of Sle (Systemic Lupus M10.1.2 73116 Erythematosis) with sepsis M10.2 Medical management of Scleroderma 30786 Medical management of Mctd Mixed Connective M10.3 25654 Tissue Disorder M10.4 Medical management of Primary Sjogren\'S Syndrome 20524 M10.5 Medical management of Vasculitis 30786 Medical management of Pyelonephritis in M11.1.1 22074 uncontrolled Diabetes melitus Medical management of Lower Respiratoy Tract M11.1.2 23603 Infection M11.1.3 Medical management of Fungal Sinusitis 42013 M11.1.4 Medical management of Cholecystitis 30479 Medical management of Cavernous Sinus Thrombosis M11.1.5 42085 in uncontrolled Diabetes melitus M11.1.6 Medical management of Rhinocerebral Mucormycosis 52973 Initial evaluation and management of Hypopituitarism M11.2.1 26681 with growth harmone M11.2.2 Hormonal therapy for Pituitary