Chilo Auricilius Dudgeon 1905 (Lepidoptera:Crambidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hymenoptera: Braconidae) (Indonesian Strain) Against Sugarcane Stalk and Internode Borers
I /Ii,,!. Coli/mi. 15(2): 127-131, 2001 Field evaluation of Cotesiaflavipes Cameron (Hymenoptera: Braconidae) (Indonesian strain) against sugarcane stalk and internode borers R. K. TANWAR and ASHOK VARMA Division of Entomology, Indian Institute of Sugarcane Research Lucknow 226 002, Uttar Pradesh, India E-mail: [email protected] ABS TRA CT: Field trials were conducted on the releases of Cotesia Jlavipes Cameron (Indonesian strain) against sugarcane stalk (Chilo auricilius Dudg.) and internode (Chilo sacchariphagus indiclls Kapur) borers at IISR Farm, Lucknow for consecutive three crop sea sons 1996-97 to 1998-99. The parasitoids were released in one block @ 2000 mated females! ha I month split into four doses from July to October and the other block was treated as check. The results indicated reduction of 56.2, 69.6 and 43.1 per cent in stalk borer infestation in parasitoid released blocks as compared to check, in October during 1996- 97, 1997-98 and 1998~99, respectively. The results remained inconclusive in cases of internode borer due to low infestation. KEY WORDS: Chilo auriciliu.f, Clli/o sacchllriplwgu.f indiclIs. Cotesia Jlavipes. parasitoid. field releases Cotesia Jlavipes Cameron (Hymenoptera: 1979) and Thailand (Suasa-ard and Charernson, Braconidae). an important gregarious larval 1999). In the recent past the Indonesian strain endoparasitoid of different sugarcane borers, has been imported to India through Project namely Chilo infuscatellus Snellen, Chilo Directorate of Biological Control, Bangalore for sacchariphagus indicus Kapur, Chilo evaluation against sugarcane borers. Laboratory tumidicostalis Hmpsn., Sesamia inferens evaluation of this strain has already been done (Walker) and Acigona steniellus (Hmpsn.) is (Tanwar and Varma, 1996). -

Downloaded from BOLD Or Requested from Other Authors
www.nature.com/scientificreports OPEN Towards a global DNA barcode reference library for quarantine identifcations of lepidopteran Received: 28 November 2018 Accepted: 5 April 2019 stemborers, with an emphasis on Published: xx xx xxxx sugarcane pests Timothy R. C. Lee 1, Stacey J. Anderson2, Lucy T. T. Tran-Nguyen3, Nader Sallam4, Bruno P. Le Ru5,6, Desmond Conlong7,8, Kevin Powell 9, Andrew Ward10 & Andrew Mitchell1 Lepidopteran stemborers are among the most damaging agricultural pests worldwide, able to reduce crop yields by up to 40%. Sugarcane is the world’s most prolifc crop, and several stemborer species from the families Noctuidae, Tortricidae, Crambidae and Pyralidae attack sugarcane. Australia is currently free of the most damaging stemborers, but biosecurity eforts are hampered by the difculty in morphologically distinguishing stemborer species. Here we assess the utility of DNA barcoding in identifying stemborer pest species. We review the current state of the COI barcode sequence library for sugarcane stemborers, assembling a dataset of 1297 sequences from 64 species. Sequences were from specimens collected and identifed in this study, downloaded from BOLD or requested from other authors. We performed species delimitation analyses to assess species diversity and the efectiveness of barcoding in this group. Seven species exhibited <0.03 K2P interspecifc diversity, indicating that diagnostic barcoding will work well in most of the studied taxa. We identifed 24 instances of identifcation errors in the online database, which has hampered unambiguous stemborer identifcation using barcodes. Instances of very high within-species diversity indicate that nuclear markers (e.g. 18S, 28S) and additional morphological data (genitalia dissection of all lineages) are needed to confrm species boundaries. -

Crambidae Biosecurity Occurrence Background Subfamilies Short Description Diagnosis
Diaphania nitidalis Chilo infuscatellus Crambidae Webworms, Grass Moths, Shoot Borers Biosecurity BIOSECURITY ALERT This Family is of Biosecurity Concern Occurrence This family occurs in Australia. Background The Crambidae is a large, diverse and ubiquitous family of moths that currently comprises 11,500 species globally, with at least half that number again undescribed. The Crambidae and the Pyralidae constitute the superfamily Pyraloidea. Crambid larvae are concealed feeders with a great diversity in feeding habits, shelter building and hosts, such as: leaf rollers, shoot borers, grass borers, leaf webbers, moss feeders, root feeders that shelter in soil tunnels, and solely aquatic life habits. Many species are economically important pests in crops and stored food products. Subfamilies Until recently, the Crambidae was treated as a subfamily under the Pyralidae (snout moths or grass moths). Now they form the superfamily Pyraloidea with the Pyralidae. The Crambidae currently consists of the following 14 subfamilies: Acentropinae Crambinae Cybalomiinae Glaphyriinae Heliothelinae Lathrotelinae Linostinae Midilinae Musotiminae Odontiinae Pyraustinae Schoenobiinae Scopariinae Spilomelinae Short Description Crambid caterpillars are generally cylindrical, with a semiprognathous head and only primary setae (Fig 1). They are often plainly coloured (Fig. 16, Fig. 19), but can be patterned with longitudinal stripes and pinacula that may give them a spotted appearance (Fig. 10, Fig. 11, Fig. 14, Fig. 22). Prolegs may be reduced in borers (Fig. 16). More detailed descriptions are provided below. This factsheet presents, firstly, diagnostic features for the Pyraloidea (Pyralidae and Crambidae) and then the Crambidae. Information and diagnostic features are then provided for crambids listed as priority biosecurity threats for northern Australia. -

Rice Package
Rice INTEGRATED PEST MANAGEMENT INNOVATION LAB rice yaleclimateconnections.org package ice is an annual, self-pollinated, and semi-aquatic plant and belongs to the family Poaceae. Asian rice (Oryza sativa; subsp. japonica and indica), WHAT IS IPM? RAfrican rice (Oryza glaberrima), and wild rice (genus Zizania) are known to be consumed by humans. Oryza sativa subsp. indica was first domesticated Integrated pest management (IPM), an in India, whereas Oryza sativa subsp. japonica was domesticated in China. Rice environmentally-sound and economical is the most important food crop in the world and is a staple food across Asia approach to pest control, was developed and becoming important in Africa and Latin America. The traditional method of in response to pesticide misuse in cultivating rice is flooding the direct-seeded fields with or after transplanting the 1960s. Pesticide misuse has led to the young seedlings and is called irrigated rice production. Rice is also grown pesticide resistance among prevailing in the rainfed lowland, in mountains or plateaus, and the deep water. About pests, a resurgence of non-target pests, loss of biodiversity, and environmental 90 percent of rice production occurs in Asia. Although rice consumption and and human health hazards. demand are increasing around the globe, especially in Asia, stability in rice production in Asia depends on social and political stability. Climate change plays a major role in rice production in Asia. Irrigated rice area provides major production, but it is hard to increase irrigated rice area because of the WHAT ARE Lab (IPM IL) Management Innovation Pest Integrated problems of soil salinity, high cost of development, water scarcity, alternative IPM PACKAGES? and competing uses of water, and environmental concerns of the emission of greenhouse gases. -

Rice Gold-Fringed Borer (410)
Pacific Pests and Pathogens - Fact Sheets https://apps.lucidcentral.org/ppp/ Rice gold-fringed borer (410) Photo 1. Adult gold-fringed rice borer, Chilo auricilius Photo 2. Adult gold-fringed rice borer, Chilo auricilius (male). (female). Photo 4. 'Whitehead' - a symptom caused by stem borers: the base of the panicle is damaged preventing it Photo 3. Damage ('deadhearts') to rice stem by Chilo from emerging or, if already emerged, the grain is auricilius. unfilled and white. Common Name Rice gold-fringed borer Scientific Name Chilo auricilius. Another species, Chilo suppressalis (the Asiatic stem borer, or striped stem borer), occurs in Australia, but not in the rest of Oceania. It is also similar to Chilo polychrysus, which occurs in India, Indonesia and Thailand. A moth in the Crambidae. Distribution Restricted. South and Southeast Asia, Oceania. It is recorded from Papua New Guinea. Hosts Rice, sugarcane, sorghum, maize, and wild grasses. Symptoms & Life Cycle The larvae do the damage by feeding on the stems and causing similar symptoms to other rice stem borers (see Fact Sheets nos. 408, 409, 411). The larvae tunnel into the stems, through the internodes towards the base of the plant, causing stems to wilt and die, a condition known as 'deadheart'. The stems are easily pulled out (Photo 3). Feeding at the base of the panicles may prevent emergence or result in white unfilled grain of those that have emerged, a symptom called 'whitehead' (Photo 4). Eggs are creamy-white, slightly flattened, scale-like, laid in 2-5 rows; they turn black later. Larvae are white, growing to 25-30 mm long with five bluish-purple lines along body, and brown heads. -
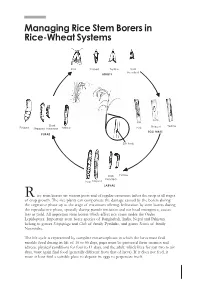
Managing Rice Stem Borers in Rice-Wheat Systems
Managing Rice Stem Borers in Rice-Wheat Systems Pink Striped Yellow Dark Headed ADULTS Dark Striped Yellow Pinked Stripped headed Yellow Pink EGG MASS PUPAE Exit hole Exit hole Dark Yellow headed Pink Striped LARVAE R ice stem borers are serious pests and of regular occurence infest the crop at all stages of crop growth. The rice plants can compensate the damage caused by the borers during the vegetative phase up to the stage of maximum tillering. Infestation by stem borers during the reproductive phase, specially during panicle initiation and ear head emergence, causes loss in yield. All important stem borers which affect rice come under the Order Lepidoptera. Important stem borer species of Bangladesh, India, Nepal and Pakistan belong to genera Scirpophaga and Chilo of family Pyralidae, and genus Sesmia of family Noctuidae. The life cycle is represented by complete metamorphosis in which the larva must find suitable food during its life of 16 to 56 days, pupa must be protected from enemies and adverse physical conditions for four to 11 days, and the adult, which lives for just two to six days, must again find food (generally different from that of larva). If it does not feed, it must at least find a suitable place to deposit its eggs to perpetuate itself. Pupation in rice stem borers usually takes place in the stem, straw, or stubble. Sometimes S. inferens also pupates between leaf sheath and stem. Pupae of Scirpophaga are covered with whitish silken cocoon, while C.suppressalis pupae are without cocoon. Before pupating, the full-grown larva cuts an exit hole in the rice internode and plugs it with a fine web through which emerging moth escapes. -

Pengaruh Lama Inokulasi Dan Ukuran Larva Chilo Sacchariphagus Boj
Jurnal Agroekoteknologi . E-ISSN No. 2337- 6597 Vol.4. No.1, Desember 2015. (565) :1741 - 1747 Pengaruh Lama Inokulasi dan Ukuran Larva Chilo sacchariphagus Boj. (Lepidoptera: Crambidae) Untuk Perbanyakan Sturmiopsis inferens Towns. (Diptera: Techinidae) di Laboratorium The Influence of Inoculation Period and Larvae Size of Chilo sacchariphagus Boj. (Lepidoptera: Crambidae) for Mass Rearing of Sturmiopsis inferens Towns. (Diptera: Techinidae) in Laboratory Citra Maharani, Maryani Cyccu Tobing*, Syahrial Oemry Program Studi Agroekoteknologi, Fakultas Pertanian, USU, Medan 20155 *Corresponding author : [email protected] ABSTRACT The objective of the research was to study the inoculation period and larvae size of Chilo sacchariphagus for mass rearing of Sturmiopsis inferens. The research was conducted in laboratory of Sugarcane Reasearch and Devolopment Sei Semayang Binjai, Medan, North Sumatera from September until October 2014 using a randomized complete design with two factors and three replications. The first factor was inoculation periods (5, 10, and 15 minutes) and the second factor was larvae sizes of C. sacchariphagus (< 1.5, 1.5 - 2 and > 2 cm). The results showed that inoculation period and larvae size significantly effected the percentage of parasititation and total of pupa. The highest percentage of parasititation (4,63 %) on inoculation period for 5 minutes with larvae size > 2 cm and the lowest (0.71 %) on inoculation period for 10 minutes with larvae size < 1.5 cm. The highest total of pupal (2.61 pupal) on inoculation period for 5 minutes with larvae size > 2 cm and the lowest (0.71 pupal) on inoculation period for 10 minutes with larvae size < 1.5 cm. The highest percentage of pupal to become imago (92.5 %) on larvae size > 2 cm and the lowest (7.5 %) on larvae size < 1.5 cm and sex ratio of male and female S. -

Rice Pink Stem Borer (409)
Pacific Pests and Pathogens - Fact Sheets https://apps.lucidcentral.org/ppp/ Rice pink stem borer (409) Photo 1. Adult pink stem borer, Sesamia inferens (underside) Photo 1. Adult pink stem borer, Sesamia inferens (top). Photo 4. 'Whitehead' - a symptom caused by stem borers: the base of the panicle is damaged preventing it Photo 3. Damage ('deadheart') to rice stem by Chilo from emerging or, if already emerged, the grain is auricilius (damage by Sesamia inferens is similar). unfilled and white. Common Name Pink stem borer; also known as the Asiatic pink stem borer or the purple stem borer. Scientific Name Sesamia inferens. A moth in the Noctuidae. Distribution Restricted. South and Southeast Asia, North America (Hawaii), Oceania. It is recorded from Guam, Papua New Guinea, and Solomon Islands. Hosts Rice, maize, millet, sorghum, sugarcane, wheat, and many grasses and some sedges, e.g., Coix lacryma-jobi (Job's tears), Echinochloa colona (jungle rice), Panicum repens (torpedo grass), Setaria pumila (yellow foxtail), and others. Symptoms & Life Cycle The larvae do the damage, similar to other species of rice borer (see Fact Sheet nos. 408, 410, 411). The larvae tunnel into the stems towards the base of the plant, causing it to wilt and die, a condition known as 'deadheart'. The stems are easily pulled out (Photo 3). Feeding at the base of the panicles may prevent emergence, or result in white unfilled grain of those that have emerged, a symptom known as 'whitehead' (Photo 4). Eggs are laid between the leaf sheaths and stem, up to 100 in rows; they are almost round, creamy-white, later turning pink and black before hatching. -

Relative Abundance of Stem Borer Species and Natural Enemies in Rice Ecosystem at Madhupur,Tangail, Bangladesh
J. Bangladesh Agril. Univ. 12(2): 267–272, 2014 ISSN 1810-3030 Relative abundance of stem borer species and natural enemies in rice ecosystem at Madhupur, Tangail, Bangladesh M. M. Rahaman*, K.S. Islam, M. Jahan and M. A. A. Mamun Department of Entomology, Bangladesh Agricultural University, Mymensigh-2202, Bangladesh, *E-mail address: [email protected] Abstract The relative abundance of different stem borer species and their natural enemies with interaction effects were studied at three growth stages of irrigated Boro rice at Madhupur under the district of Tangail, Bangladesh during January to April, 2013. Five stem borer species viz; Yellow stem borer (Scirpophaga incertulas), Pink stem borer (Sesamia inferens), Dark headed stem borer (Chilo polyhcrysus), Stripped stem borer (Chilo suppressalis), White stem borer (Scirpophaga innotata), and nine different natural enemies were collected from the rice fields and recorded. The population of stem borers and natural enemies was highest in tillering stage and lowest in seedling stage. The relative abundance of stem borer species under investigation showed ranking order; yellow stem borer >dark headed stem borer>pink borer>white borer>stripped stem borer and natural enemies as ladybird beetle >long jawed spider>wolf spider>damselfly>carabid beetle>green mirid bug>lynx spider>dragon fly>ear wig. Populations of all five stem borers were positively correlated with ladybird beetle, wolf spider, long jawed spider, lynx spider, damsel fly, dragon fly, green mirid bug and negatively correlated with carabid beetle and earwig. Keywords: Stem borers, Natural enemies, Relative abundance, Rice ecosystem Introduction Rice (Oryza sativa L.) is one of the world’s most important crops providing a staple food for nearly half of the global population (FAO, 2004). -

Diversity of Insect Pest and Predator Species in Monsoon and Summer Rice Fields of Taungoo Environs, Myanmar
Advances in Entomology, 2020, 8, 117-129 https://www.scirp.org/journal/ae ISSN Online: 2331-2017 ISSN Print: 2331-1991 Diversity of Insect Pest and Predator Species in Monsoon and Summer Rice Fields of Taungoo Environs, Myanmar San San Oo1*, Khin Myat Hmwe1, Nyo Nyo Aung1, Aye Aye Su1, Khin Khin Soe1, Tin Lay Mon1, Khin Mar Lwin2, May Myat Thu3, Thin Thin Soe2, Myat Lwin Htwe2 1Zoology Department, University of Yangon, Yangon, Myanmar 2Zoology Department, Kyaing Tong University, Kyaing Tong, Myanmar 3Zoology Department, Dagon University, Yangon, Myanmar How to cite this paper: Oo, S.S., Hmwe, Abstract K.M., Aung, N.N., Su, A.A., Soe, K.K., Mon, T.L., Lwin, K.M., Thu, M.M., Soe, Paddy fields are natural and artificial wetland ecosystems that supply rice for T.T. and Htwe, M.L. (2020) Diversity of the people and provide the wildlife especially insect diversity of different Insect Pest and Predator Species in Mon- functional aspects. A total of 71 insect species belonging to 40 families under soon and Summer Rice Fields of Taungoo Environs, Myanmar. Advances in Ento- eight orders were observed during the study period. Among the 71 insect mology, 8, 117-129. species, 18 species of beetles, nine species of bugs, eight species of dragonfly, https://doi.org/10.4236/ae.2020.83009 five species of butterflies, four species of leafhoppers, plant hoppers and moths, three borer and spiders, two crickets, one species of skippers, grass Received: May 13, 2020 hopper, hispa, ant, weevil, hairy caterpillar, leaf roller, katydid, thrips, maggot Accepted: May 31, 2020 Published: June 3, 2020 and water boatmen were recorded in the study sites. -

Chilo Partellus
CAPS Pest Datasheets provide pest-specific information to support planning and completing early detection surveys. Chilo partellus Scientific Name Chilo partellus (Swinhoe, 1886) Synonyms: Chilo zonellus (Swinhoe, 1884) Crambus zonellus Swinhoe, 1885 Crambus partellus Swinhoe, 1885 Argyria lutulentalis Tams, 1932 Chilo lutulentalis Tams, 1932 Common Name Spotted stem borer, spotted stemborer, Figure 1. Adult female Chilo partellus moth. maize and jowar borer, maize stem borer, Photo by Paul-Andre Calatayud, IRD-ICIPE, Nairobi. Used with permission. durra stalk borer, spotted stalk borer, spotted sorghum stem borer, pink borer Type of Pest Moth, stem borer Taxonomic Position Class: Insecta, Order: Lepidoptera, Family: Crambidae Reason for Inclusion in Manual Priority pest for Corn Commodity Survey, 2017 - present Pest Description Eggs: Eggs are flat, scale-like, and cream colored (Whittle and Ferguson, 1988). They are laid in clusters of 10 to 80 (Harris, 1990) and darken in color as they develop (Fig. 2). Larvae: Caterpillars are cream-colored with four longitudinal stripes (Meijerman and Ulenberg, 1998) (Fig. 3). Diapausing larvae may not have Figure 2. Stages of egg development: i. stripes. Larvae have obvious dark brown spots newly laid. ii. brownheads. iii. blackheads. on their backs (Meijerman and Ulenberg, 1998). Photo by Dr. K. V. Seshu Reddy. Used The head capsule, prothoracic shield, and anal with permission. Version 1 June 14, 2019 1 shield are brown. The crochets1 on the prolegs2 form a circle with three alternating lengths in some parts. Spiracles are black and oval-shaped (Meijerman and Ulenberg, 1998). Pupae: The edges of abdominal segments 5, 6, and 7 have rough patches (Whittle and Ferguson, 1988) and the last segment of the abdomen has eight to nine prominent points (Whittle and Ferguson, 1988). -

6. Stem Borers of Gramineous Crops in Southeast Asia
145 6. STEM BORERS OF GRAMINEOUS CROPS IN SOUTHEAST ASIA Isoko HATTORI* Major Stem Borers Attacking Gramineous Crops in Southeast Asia More than a dozen species of stem borers are known as injurious pests of cereals and sugarcane in Southeast Asia. Names of major stem borers are given below in Table 1, and their host plants and distribution are shown in Table 2. Table 1. The list of major stem borers of gramineous crops in Southeast Asia. Species Scientific names formerly used Common name Chilo suppressalis (Walker) Chilo simplex (Butler) rice stem borer Chilo oryzae (Fletcher) striped stalk borer Chilo tumidicostalis (Hampson) Argyria tumidicostalis Hampson stem borer (India) Chilo partellus (Swinhoe) Chilo simplex (Butler) stem borer (India) Chilo zonellus Swinhoe maize borer (India) Argyria lutulentalis Tams Chilo auricilius Dudgeon Chilo auricilia Dudgeon stem borer Diatraea auricilia (Dudgeon) stalk borer (India) Chilotraea auricilia (Dudgeon) Chilo polychrysus Meyrick Diatraea polychrysa Meyrick paddy borer Proceras polychrysa (Meyrick) paddy stem borer Diatraea auricilia (Dudgeon) Chilotraea polychrysa (Dudgeon) Chilo infuscatellus Snellen Argyria sticticraspis Hampson early shoot borer (India) Argyria coniorta Hampson shoot borer (India) Chilotraea infuscatella (Snellen) yellow top borer (Java) Diatraea shariinensis (Eguchi) Chilo sacchariphagus Proceras sacchariphagus Bojer striped stalk borer (Java) sacchariphagus (Bojer) Diatraea striatalis Snellen spotted borer (Mauritius) Diatraea venosata (Walker) Diatraea 1nauriciella (Walker)