Dynamics and Quantification of Dissolved Metals in a Highly Contaminated River-Estuarine System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Multi- Hazard District Disaster Management Plan
MULTI –HAZARD DISTRICT DISASTER MANAGEMENT PLAN, BIRBHUM 2018-2019 MULTI – HAZARD DISTRICT DISASTER MANAGEMENT PLAN BIRBHUM - DISTRICT 2018 – 2019 Prepared By District Disaster Management Section Birbhum 1 MULTI –HAZARD DISTRICT DISASTER MANAGEMENT PLAN, BIRBHUM 2018-2019 2 MULTI –HAZARD DISTRICT DISASTER MANAGEMENT PLAN, BIRBHUM 2018-2019 INDEX INFORMATION 1 District Profile (As per Census data) 8 2 District Overview 9 3 Some Urgent/Importat Contact No. of the District 13 4 Important Name and Telephone Numbers of Disaster 14 Management Deptt. 5 List of Hon'ble M.L.A.s under District District 15 6 BDO's Important Contact No. 16 7 Contact Number of D.D.M.O./S.D.M.O./B.D.M.O. 17 8 Staff of District Magistrate & Collector (DMD Sec.) 18 9 List of the Helipads in District Birbhum 18 10 Air Dropping Sites of Birbhum District 18 11 Irrigation & Waterways Department 21 12 Food & Supply Department 29 13 Health & Family Welfare Department 34 14 Animal Resources Development Deptt. 42 15 P.H.E. Deptt. Birbhum Division 44 16 Electricity Department, Suri, Birbhum 46 17 Fire & Emergency Services, Suri, Birbhum 48 18 Police Department, Suri, Birbhum 49 19 Civil Defence Department, Birbhum 51 20 Divers requirement, Barrckpur (Asansol) 52 21 National Disaster Response Force, Haringahata, Nadia 52 22 Army Requirement, Barrackpur, 52 23 Department of Agriculture 53 24 Horticulture 55 25 Sericulture 56 26 Fisheries 57 27 P.W. Directorate (Roads) 1 59 28 P.W. Directorate (Roads) 2 61 3 MULTI –HAZARD DISTRICT DISASTER MANAGEMENT PLAN, BIRBHUM 2018-2019 29 Labpur -

Eastern India Pramila Nandi
P: ISSN NO.: 2321-290X RNI : UPBIL/2013/55327 VOL-5* ISSUE-6* February- 2018 E: ISSN NO.: 2349-980X Shrinkhla Ek Shodhparak Vaicharik Patrika Dimension of Water Released for Irrigation from Mayurakshi Irrigation Project (1985-2013), Eastern India Abstract Independent India has experienced emergence of many irrigation projects to control the river water with regulatory measures i.e. dam, barrage, embankment, canal etc. These irrigation projects were regarded as tools of development and it was thought that they will take the economy of the respective region to a higher level. Against this backdrop, the Mayurakshi Irrigation Project was initiated in 1948 with Mayurakshi as principal river and its four main tributaries namely Brahmani, Dwarka, Bakreswar and Kopai. This project aimed to supply water for irrigation to the agricultural field of the command area at the time of requirement and assured irrigation was the main agenda of this project’s commencement. In this paper the author has tried to find out the current status of the timely irrigation water supply which was the main purpose of initiation of this project. Keywords: Irrigation Projects, Regulatory Measures, Command Area, Assured Irrigation. Introduction In the post-independence period, India has shown accelerating trend in growth of irrigation projects. Following USA and other advanced economies of the time, independent India encouraged irrigation projects to ensure assured irrigation, flood control, generation of hydroelectricity. Then Prime Minister Jawhar Lal Neheru entitled the dams as temples of modern India. Mayurakshi Irrigation Project (MIP) was one of them and was Pramila Nandi launched in 1948 to serve water to the thirsty agricultural lands of one of Research Scholar, the driest district of West Bengal i.e. -

The National Waterways Bill, 2016
Bill No. 122-F of 2015 THE NATIONAL WATERWAYS BILL, 2016 (AS PASSED BY THE HOUSES OF PARLIAMENT— LOK SABHA ON 21 DECEMBER, 2015 RAJYA SABHA ON 9 MARCH, 2016) AMENDMENTS MADE BY RAJYA SABHA AGREED TO BY LOK SABHA ON 15 MARCH, 2016 ASSENTED TO ON 21 MARCH, 2016 ACT NO. 17 OF 2016 1 Bill No. 122-F of 2015 THE NATIONAL WATERWAYS BILL, 2016 (AS PASSED BY THE HOUSES OF PARLIAMENT) A BILL to make provisions for existing national waterways and to provide for the declaration of certain inland waterways to be national waterways and also to provide for the regulation and development of the said waterways for the purposes of shipping and navigation and for matters connected therewith or incidental thereto. BE it enacted by Parliament in the Sixty-seventh Year of the Republic of India as follows:— 1. (1) This Act may be called the National Waterways Act, 2016. Short title and commence- (2) It shall come into force on such date as the Central Government may, by notification ment. in the Official Gazette, appoint. 2 Existing 2. (1) The existing national waterways specified at serial numbers 1 to 5 in the Schedule national along with their limits given in column (3) thereof, which have been declared as such under waterways and declara- the Acts referred to in sub-section (1) of section 5, shall, subject to the modifications made under this tion of certain Act, continue to be national waterways for the purposes of shipping and navigation under this Act. inland waterways as (2) The regulation and development of the waterways referred to in sub-section (1) national which have been under the control of the Central Government shall continue, as if the said waterways. -

NW-22 Birupa Badi Genguti Brahmani Final
Final Feasibility Report of Cluster 4 – Birupa / Badi Genguti / Brahmani River Feedback Infra (P) Limited i Final Feasibility Report of Cluster 4 – Birupa / Badi Genguti / Brahmani River Table of Content 1 Executive Summary ......................................................................................................................... 1 2 Introduction ..................................................................................................................................... 7 2.1 Inland Waterways in India ...................................................................................................... 7 2.2 Project overview ..................................................................................................................... 7 2.3 Objective of the study ............................................................................................................. 7 2.4 Scope ....................................................................................................................................... 8 2.4.1 Scope of Work in Stage 1 .................................................................................................... 8 2.4.2 Scope of Work in Stage 2 .................................................................................................... 8 3 Approach & Methodology ............................................................................................................. 11 3.1 Stage-1 ................................................................................................................................. -

Notice Inviting E-Auction for Grant of Mining Lease of Sand
Government of West Bengal Office of the District Land & Land Reforms Officer Murshidabad Memo. No. 7189 /X-34/C/17 Dated, Berhampore, the 17th August, 2017 Notice inviting e-Auction for grant of Mining Lease of Sand Notices are hereby invited for e-Auction from bona fide individuals, firms and companies for grant of mining lease of sand (minor minerals) only through online mode of bidding in the Government e-Auction System (http:// eauction.gov.in) for 11 (eleven) nos. Sand Blocks situated in District- Murshidabad and Block-M-J, Raghu-II & Nabagram in the riverbed of Dwarka, Bhairav, Bhagirathi / Padma & Brahmani river as per provisions of the West Bengal Minor Minerals (Concession) Rules, 2016 and West Bengal Minor Minerals (Auction) Rules, 2016, whose land schedules and maps are annexed with this notice. The terms and conditions are given in the Bid document annexed with this notice and also in the Government e-Auction System Portal. It is also available at the offices of the: 1) District Magistrate & Collector, Murshidabad. 2) District Land & Land Reforms Office, Murshidabad. 3) Sub-Divisional Land & Land Reforms Office (all), Murshidabad. 4) Block Land & Land Reforms Office (all), Murshidabad Intending bidders must have Digital Signature Certificate for e-submission of their bids online through e- Procurement Portal & to participate in the competitive bidding online. Relevant papers / documents (listed below) should be submitted in any working day between 11 a.m. to 3 p.m. on and from 21/08/2017 to 23/08/2017 at the sealed tender box kept in the Office of the Additional District Magistrate (LR) & District Land & Land Reforms Officer, Murshidabad. -

Water Quality Assessment of Brahmani River at Talcher City, Odisha (A Case Study)
IOSR Journal of Mechanical and Civil Engineering (IOSR-JMCE) e-ISSN: 2278-1684,p-ISSN: 2320-334X, Volume 15, Issue 5 Ver. IV (Sep. - Oct. 2018), PP 25-33 www.iosrjournals.org Water Quality Assessment of Brahmani River At Talcher City, Odisha (A Case Study) Chanchal Kumar Mukherjee1, Dr.Bhagirathi Tripathy2, Dr. P K Pani3, Abhijeet Das4 1 Research Scholar, Utkal University, 2Assistant Professor, Civil Engineering Department, IGIT, Sarang, Odisha. 3 Professor, Civil Engineering Department, IGIT, Sarang, Odisha. 4 Assistant Professor, Civil Engineering Department, IGIT, Sarang, Odisha. Corresponding Author: Chanchal Kumar Mukherjee Abstract: Water, food, energy and the environment have got intertwined in a spiral of decline and degradation .The challenge is to slow the spin and reverse the direction. The world’s thirst for water is likely to become one of the most pressing issues of the 21st century. Rapid pace of industrialization, concurrent growth of urbanization, need and change of life style of ever expanding population have the potential to damage the environment and degrade the available surface water sources. Since there has been growing concern about pollution in Talcher area due to industrial, mining and other anthropogenic activities, Central Pollution Control Board and Ministry of Environment & Forests have identified this zone as one of the hot spots in respect of pollution hazards. The present investigation deals with a comparative study of physico-chemical characteristics of water samples taken from four different sampling locations situated near the industrial zone of Talcher near Brahmani basin. The parameters were constantly monitored like pH, conductivity, hardness, DO, BOD, COD, TDS, TSS, Phosphate, Sulphate, Nitrate, Chloride etc. -

Dams, Rivers & People
Dams, Rivers & People SANDRP VOL 2-ISSUE 5-6 Rs 15/- JUNE-JULY 2004 INDEX ADB money for Bisalpur project, but not to DAM! 30 Another dam, displacement without rehabiliation 2 Flood News: Is it the worst flood 31 Civil Society Position Paper on WB CAS 3 PM’s task force on floods 32 Garland of Hype: Response to Suresh Prabhu Mockery of flood assistance in Bihar Dunu Roy 4 Dinesh Kumar Mishra 33 River Link News: SC Orders Violated 5 How fishing ban helps Fish conservation in HP Bhakra at risk: Silt Delta at Gobind Sagar 6 Kuldip Kumar Sharma 34 Case for decommissioning of Dumbur Dam 7 And yet fish habitats deplete in HP: 35 Gosikhurd Dam Oustees Protest 8 Soils of India 36 Villages under threat due to Parbati HEP in HP 9 Rajasthan to legislate FOR contract farming 37 NHPC faces protests over Middle Siang HEP 10 Virtual Water Trade: India among top exporters 38 NHPC violations in TEESTA V 11 Power news 39 Kerala PH Rejects Silent Valley Project 12 Power Finance News 40 SC directs MP to provide land based R&R 13 Power Options: Renewables in India 41 Costly Narmada Bonds 14 AP HC Bats for Renewables 42 Polluted Rivers: Sutlej 15 No State is Power Surplus: Assocham 43 No HEPs in Banjar Valley? 16 CAG indicts Delhi, Maharashtra: Who cares? 44 W Bengal’s bill to imperil water bodies 17 2.88% in Nepal have renewable energy 45 Climate to cause water scarcity in India? 18 Pakistan: GMof HEP sentenced to RI 46 Water Privatisation is not the answer says UN 19 BD: UN urged to review water convention 47 New Krishna Tribunal challenged by Karnataka 20 -

Geography of World and India
MPPSCADDA 1 GEOGRAPHY OF WORLD AND INDIA CONTENT WORLD GEOGRAPHY ❖ ❖ ❖ ❖ ❖ ❖ ❖ ❖ ❖ INDIAN GEOGRAPHY ❖ ❖ ❖ ❖ ❖ ❖ 2 MPPSCADDA 3 GEOGRAPHY WORLD 1. UNIVERSE INTRODUCTION TO GEOGRAPHY • The word ‘Geography’ is a combination of two Greek words "geo" means Earth and "graphy" means write about. • Geography as a subject not only deals with the features and patterns of surface of Earth, it also tries to scientifically explain the inter-relationship between man and nature. • In the second century, Greek scholar Eratosthenes (Father of Geography) adopted the term 'Geography'. BRANCHES OF GEOGRAPHY Physical Geography Human Geography Bio - Geography Cultural Geography Climatology Economic Geography Geomorphology Historical Geography Glaciology Political Geography Oceanography Population Geography Biogeography Social Geography Pedology Settlement Geography PHYSICAL GEOGRAPHY It deals with the physical environment and various processes that bring about changes in the physical environment on the Earth's surface. It includes: 1. Bio-Geography: The study of the geographic distribution of organisms. 2. Climatology: The study of climate or weather conditions averaged over a period of time. 3. Geomorphology or Physiographic: The scientific study of landforms and processes that shape them. 4. Glaciology: The study of glaciers and ice sheets. 5. Oceanography: The study of all aspects of the ocean including temperature, ocean current, salinity, fauna and flora, etc. 6. Pedology: The study of various types of Soils. 4 HUMAN GEOGRAPHY Human geography deals with the perspective of human and its functions as well as its interaction with the environment. It studies people, communities and cultures with an emphasis on relations of land across space. It includes: 1. Cultural Geography: The study of the spatial variations among cultural groups and spatial functioning of the society. -
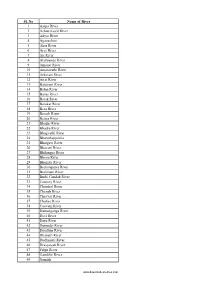
List of Rivers in India
Sl. No Name of River 1 Aarpa River 2 Achan Kovil River 3 Adyar River 4 Aganashini 5 Ahar River 6 Ajay River 7 Aji River 8 Alaknanda River 9 Amanat River 10 Amaravathi River 11 Arkavati River 12 Atrai River 13 Baitarani River 14 Balan River 15 Banas River 16 Barak River 17 Barakar River 18 Beas River 19 Berach River 20 Betwa River 21 Bhadar River 22 Bhadra River 23 Bhagirathi River 24 Bharathappuzha 25 Bhargavi River 26 Bhavani River 27 Bhilangna River 28 Bhima River 29 Bhugdoi River 30 Brahmaputra River 31 Brahmani River 32 Burhi Gandak River 33 Cauvery River 34 Chambal River 35 Chenab River 36 Cheyyar River 37 Chaliya River 38 Coovum River 39 Damanganga River 40 Devi River 41 Daya River 42 Damodar River 43 Doodhna River 44 Dhansiri River 45 Dudhimati River 46 Dravyavati River 47 Falgu River 48 Gambhir River 49 Gandak www.downloadexcelfiles.com 50 Ganges River 51 Ganges River 52 Gayathripuzha 53 Ghaggar River 54 Ghaghara River 55 Ghataprabha 56 Girija River 57 Girna River 58 Godavari River 59 Gomti River 60 Gunjavni River 61 Halali River 62 Hoogli River 63 Hindon River 64 gursuti river 65 IB River 66 Indus River 67 Indravati River 68 Indrayani River 69 Jaldhaka 70 Jhelum River 71 Jayamangali River 72 Jambhira River 73 Kabini River 74 Kadalundi River 75 Kaagini River 76 Kali River- Gujarat 77 Kali River- Karnataka 78 Kali River- Uttarakhand 79 Kali River- Uttar Pradesh 80 Kali Sindh River 81 Kaliasote River 82 Karmanasha 83 Karban River 84 Kallada River 85 Kallayi River 86 Kalpathipuzha 87 Kameng River 88 Kanhan River 89 Kamla River 90 -

Mineralogy and Geochemistry of the Sediments in Rivers Along the East Coast of India: Inferences on Weathering and Provenance
J. Earth Syst. Sci. (2021) 130:60 Ó Indian Academy of Sciences https://doi.org/10.1007/s12040-020-01551-5 (0123456789().,-volV)(0123456789().,-volV) Mineralogy and geochemistry of the sediments in rivers along the east coast of India: Inferences on weathering and provenance 1 1, 1 SHAIK SAI BABU ,VENIGALLA PURNACHANDRA RAO *, NANNAPANENI SATYASREE , 1 2 2 RAVIPATI VENKATA RAMANA ,MEKALA RAM MOHAN and SARIPOOT SAWANT 1Vignan’s Foundation for Science, Technology and Research (VFSTR), Deemed to be Vignan’s University, Vadlamudi, Guntur 522 213, India. 2CSIR-National Geophysical Research Institute, Uppal Road, Hyderabad 500 007, India. *Corresponding author. e-mail: [email protected] MS received 25 July 2020; revised 19 November 2020; accepted 25 November 2020 The clay fraction of sediments in the lower reaches of 15 rivers along the east coast of India showed high kaolinite followed by illite and smectite for the rivers dominantly draining the Archaean–Precambrian Terrain (APT), high smectite followed by illite and kaolinite for those draining the Deccan Trap Volcanic Terrain (DVT) and, high illite followed by kaolinite, smectite or chlorite for those draining through Mixed-Lithology Terrain (MLT). The CIA (Chemical Index of Alteration) and PIA (Plagioclase Index of Alteration) values, depletion of Ca, K, Na and Sr and enrichment of Al, Fe and Ti, high Rb/Sr and Th/U ratios of sediments relative to the Upper Continental Crust indicate moderate to intense chemical weathering on source rocks. The average composition of clays exhibits slight enrichment of Fe, Mg, Sc, V, Co, Cr and Ni and depletion of Nb, Zr, Hf, Y and Ta relative to the Post-Archaean average Australian Shale. -

ANSWERED ON:03.03.2016 Development of Inland Waterways Ering Shri Ninong;Rajendran Shri S.;Singh Dr
GOVERNMENT OF INDIA SHIPPING LOK SABHA UNSTARRED QUESTION NO:1264 ANSWERED ON:03.03.2016 Development of Inland Waterways Ering Shri Ninong;Rajendran Shri S.;Singh Dr. Bhola Will the Minister of SHIPPING be pleased to state: (a) the details of targets set and progress made in the development of inland waterways in the country and the roadblocks identified in this regard alongwith the steps taken by the Government to remove them; (b) the details and status of Sagar Mala project for development of waterways transport in the country; (c) whether the Government has prepared any scheme for the development of inland waterways as National Waterways; (d) if so, the names of rivers declared as National Waterways in the country and the extent and scope of each of these waterways, State/ UT-wise including Goa; and (e) the funds allocated/ proposed to be allocated for the development of these waterways and the types of development envisaged for the National Waterways in various States including Goa? Answer MINISTER OF STATE IN THE MINISTRY OF SHIPPING (SHRI PON. RADHAKRISHNAN) (a): The following five waterways are declared as National Waterways (NWs) so far: i. Ganga-Bhagirathi-Hooghly river system (Allahabad-Haldia-1620 km) as NW-1 ii. River Brahmaputra (Dhubri-Sadiya − 891 km) as NW-2. iii. West Coast Canal (Kottapuram-Kollam) along with Udyogmandal and Champakara Canals − (205 km) as NW-3. iv. Kakinada- Puducherry canals along with Godavari and Krishna rivers (1078 km) as NW-4. v. East Coast Canal integrated with Brahmani river and Mahanadi delta rivers (588 km) as NW-5. -

A Case Study of Flood in 2000 in Murshidabad, West Bengal
Research Paper Volume : 2 | Issue : 3 |Geography Mar 2013 • ISSN No 2277 - 8179 Role of Reservoirs in Flood: A Case Study KEYWORDS : Flood in Murshidabad, of Flood in 2000 in Murshidabad, West Massanjore Dam, Mayurakshi River Bengal Basin, Damodar Valley Corporation Swati Mollah Asst. Prof. in Geography, Department of Geography, Dumkal College, P.O. Basantapur, Dumkal, Murshidabad, PIN- 742 406 ABSTRACT In India, for controlling flood emphasis has been on structural measures especially construction of multipur- pose projects due to economy of construction cost. They are required to moderate the incoming floods to the maximum possible extent as well as store enough water during the monsoon period so that the conservation purpose are best served during the remaining period of the year. But unfortunately these structural measures have been proved futile for flood management. The main problem of these reservoirs is heavy siltation and inappropriate maintenance. In most cases, the actual rate of siltation is found to be higher than the design rate. For the 23 reservoirs the annual loss in live storage capacity is 0.912% of the original live storage capacity. These 23 reservoirs have already lost 23.11 % of LS by 2006. This paper attempts to find out the problems regarding the Massanjore Dam in controlling flood in Murshidabad, in particular based on secondary data from various government reports. It may help the administration of the district to find the solution of the flood problem in other way than depending on controlling flood through reservoir regulation. INTRODUCTION Most parts of India being dependent for water on 3-4 month long monsoon, reservoirs are created to store water for use oftrolling 2000 flood.in Murshidabad and its adjoining parts.