Female Salix Viminalis Are More Severely Infected by Melampsora Spp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
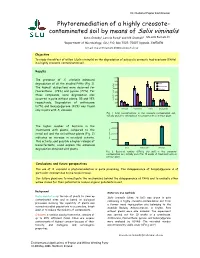
Phytoremediation of a Highly Creosote- Contaminated Soil by Means of Salix Viminalis
International Poplar Commission Phytoremediation of a highly creosote- contaminated soil by means of Salix viminalis Karin Önneby1, Leticia Pizzul1 and Ulf Granhall1 Mauritz Ramstedt 1Department of Microbiology, SLU, P.O. Box 7025, 75007 Uppsala, SWEDEN Email:E-mail: mauritz.ramstedt@m [email protected] Objective To study the effect of willow (Salix viminalis) on the degradation of polycyclic aromatic hydrocarbons (PAHs) in a highly creosote-contaminated soil. Results 1600 The presence of S. viminalis enhanced 1400 Initial degradation of all the studied PAHs (Fig. 1). 1200 The highest dissipations were observed for Without plant 1000 With plant fluoranthene (79%) and pyrene (77%). For 800 those compounds, some degradation also 600 occurred in pots without plants, 35 and 19% 400 Concentration (mg/kg dw) Concentration respectively. Degradation of anthracene 200 (67%) and benzo[a]pyrene (43%) was found 0 Anthracene Fluoranthene Pyrene Benzo[a]pyrene only in pots with S. viminalis. Fig. 1. PAH concentrations in the creosote-contaminated soil, initially and after 10 weeks of treatment with or without plant. 4000000 The higher number of bacteria in the 3000000 treatments with plants, compared to the initial soil and the soil without plants (Fig. 2) 2000000 indicates an increase in microbial activity. CFU (dw) /g soil 1000000 This activity, and possibly a higher release of biosurfactants, could explain the enhanced 0 degradation obtained with plants. Initial Without plant With plant Fig. 2. Bacterial number (CFU/g dry soil) in the creosote- contaminated soil, initially and after 10 weeks of treatment with or without plant. Conclusions and future perspectives The use of S. -

Cultivated Willows Would Not Be Appropriate Without Mention of the ‘WEEPING WILLOW’
Alexander Robertson Notes on willows cultivated in Scotland and a few sketches of willows sampled from my clonal collection HISTORICAL NOTES Since the knowledge of willows is of great antiquity, it is with the ancient Greeks and Romans we shall begin, for among these people numerous written records remain. The growth habit, ecology, cultivation and utilization of willows was well— understood by Theophrastus, Ovid, Herodotus, Pliny and Dioscorides. Virgil was also quite familiar with willow, e.g. Damoetas complains that: “Galatea, saucy girl, pelts me with apples and then runs off to the willows”. ECLOGIJE III and of foraging bees: “Far and wide they feed on arbutus, pale-green willows, on cassia and ruddy crocus .. .“ GEORGICS IV Theophrastus of Eresos (370—285 B.C.) discussed many aspects of willows throughout his Enquiry into Plants including habitats, wood quality, coppicing and a variety of uses. Willows, according to Theophrastus are lovers of wet places and marshes. But he also notes certain amphibious traits of willows growing in mountains and plains. To Theophrastus they appeared to possess no fruits and quite adequately reproduced themselves from roots, were tolerant to flooding and frequent coppicing. “Even willows grow old and when they are cut, no matter at what height, they shoot up again.” He described the wood as cold, tough, light and resilient—qualities which made it useful for a variety of purposes, especially shields. Such were the diverse virtues of willow that he suggested introducing it for plant husbandry. Theophrastus noted there were many different kinds of willows; three of the best known being black willow (Salix fragilis), white willow (S. -

List of Publications —MARIA GREGER
List of Publications —MARIA GREGER Scientific Publications (Paper with review system = R; Included in thesis = X) 1. RX Greger M. & Lindberg S., 1986. Effects of Cd2+ and EDTA on young sugar beets (Beta vulgaris). I. Cd2+uptake and sugar accumulation. — Physiologia Plantarum 66: 69-74. 2. RX Greger M. & Lindberg S., 1987. Effects of Cd2+ and EDTA on young sugar beets (Beta vulgaris). II. Net uptake and distribution of Mg2+, Ca2+ and Fe2+/Fe3+. — Physiologia Plantarum 69: 81-86. 3. RX Greger M., 1989. Cadmium Effects on Carbohydrate Metabolism in Sugar Beet (Beta vulgaris). — Thesis, Stockholm University, ISBN 91-7146-717-3 4. R Kronestedt-Robards E. C., Greger M. & Robards A. W., 1989. The nectar of the Strelizia Reginæ flower. — Physiologia Plantarum 77: 341-346. 5. R X Greger M., Brammer E. S., Lindberg S., Larsson G. & Idestam-Almquist J., 1991. Uptake and physiological effects of cadmium in sugar beets (Beta vulgaris) related to mineral provision. — Journal of Experimental Botany 42: 729-737. 6. R Lindberg S., Szynkier K. & Greger M., 1991. Aluminum effects on transmembrane potential in cells of fibrous roots of sugar beet. — Physiologia Plantarum 83: 54-62. 7. R X Greger M. & Ögren E., 1991. Direct and indirect effects of Cd2+ on the photosynthesis and CO2-assimilation in sugar beets (Beta vulgaris). — Physiologia Plantarum 83: 129-135. 8. R Greger M. & Kautsky L., 1991. Effects of Cu, Pb, and Zn on two species of Potamogeton grown in field conditions. — Vegetatio 97: 173-184. 9. R X Greger M. & Bertell G., 1992. Effects of Ca2+ and Cd2+ on the carbohydrate metabolism in sugar beet (Beta vulgaris). -

Phytoremediation of Metal Contamination Using Salix (Willows)
University of Denver Digital Commons @ DU Electronic Theses and Dissertations Graduate Studies 1-1-2015 Phytoremediation of Metal Contamination Using Salix (Willows) Gordon J. Kersten University of Denver Follow this and additional works at: https://digitalcommons.du.edu/etd Part of the Ecology and Evolutionary Biology Commons, and the Other Environmental Sciences Commons Recommended Citation Kersten, Gordon J., "Phytoremediation of Metal Contamination Using Salix (Willows)" (2015). Electronic Theses and Dissertations. 1034. https://digitalcommons.du.edu/etd/1034 This Thesis is brought to you for free and open access by the Graduate Studies at Digital Commons @ DU. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ DU. For more information, please contact [email protected],[email protected]. Phytoremediation of Metal Contamination using Salix (willows) ______________________ A Thesis Presented to The Faculty of Natural Sciences and Mathematics University of Denver ______________________ In Partial Fulfillment Of the Requirements for the Degree Master of Science ______________________ By Gordon J. Kersten August 2015 Advisor: Martin F. Quigley Author: Gordon J. Kersten Title: Phytoremediation of Metal Contamination using Salix (willows) Advisor: Martin F. Quigley Degree Date: August 2015 ABSTRACT Abandoned hardrock mines and the resulting Acid Mine Drainage (AMD) are a source of vast, environmental degradation that are toxic threats to plants, animals, and humans. Cadmium (Cd) and lead (Pb) are metal contaminants often found in AMD. In my mine outwash water samples, cadmium and lead concentrations were 19 and 160 times greater than concentrations in control waterways, and 300 and 40 times greater than EPA Aquatic Life Use water quality standards, respectively. -

Fine Mapping of the Sex Locus in Salix Triandra Confirms a Consistent Sex Determination Mechanism in Genus Salix
Li et al. Horticulture Research (2020) 7:64 Horticulture Research https://doi.org/10.1038/s41438-020-0289-1 www.nature.com/hortres ARTICLE Open Access Fine mapping of the sex locus in Salix triandra confirms a consistent sex determination mechanism in genus Salix Wei Li1,HuaitongWu1, Xiaoping Li1, Yingnan Chen1 andTongmingYin1 Abstract Salix triandra belongs to section Amygdalinae in genus Salix, which is in a different section from the willow species in which sex determination has been well studied. Studying sex determination in distantly related willow species will help to clarify whether the sexes of different willows arise through a common sex determination system. For this purpose, we generated an intraspecific full-sib F1 population for S. triandra and constructed high-density genetic linkage maps for the crossing parents using restriction site-associated DNA sequencing and following a two-way pseudo-testcross strategy. With the established maps, the sex locus was positioned in linkage group XV only in the maternal map, and no sex linkage was detected in the paternal map. Consistent with previous findings in other willow species, our study showed that chromosome XV was the incipient sex chromosome and that females were the heterogametic sex in S. triandra. Therefore, sex in this willow species is also determined through a ZW sex determination system. We further performed fine mapping in the vicinity of the sex locus with SSR markers. By comparing the physical and genetic distances for the target interval encompassing the sex determination gene confined by SSRs, severe recombination repression was revealed in the sex determination region in the female map. -

Shrub Willow Biomass Producer's Handbook
ShrubShrub WillowWillow BiomassBiomass Producer’sProducer’s HandbookHandbook Planting willow with Step Planter 3-year old willow — ready to harvest Harvesting willow with New Holland forage harvester and willow cutting head One-year old willow Willow catkins SHRUB WILLOW BIOMASS PRODUCER’S HANDBOOK PAGE 2 Shrub Willow Biomass Producer’s Handbook Lawrence P. Abrahamson, Senior Research Associate Timothy A. Volk, Senior Research Associate Lawrence B. Smart1, Associate Professor Kimberly D. Cameron1, Research Scientist State University of New York College of Environmental Science and Forestry 1 Forestry Dr. Syracuse, NY 13210 Revised December 2010 1 now at Cornell University in the Department of Horticultural Sciences, New York State Agricultural Experiment Station in Geneva, NY PAGE 3 SHRUB WILLOW BIOMASS PRODUCER’S HANDBOOK Table of Contents I. Preface……………………………………………………………………………. 4 II. Introduction……………………………………………….………………………. 5 a. Benefits for Growing Willow…………………………………………….. 5 i. Harvesting for Sale to the Energy Market………………………... 5 ii. Incentive Programs for Farmers………………………….. 5 iii. Environmental Benefits and Sustainability………………. 6 b. Cultivation of Shrub Willow: History and Current Prospects……………. 6 III. Soil & Site Considerations for Willow Biomass Crops …………………………. 8 IV. Growing Willow………………………………………………………………... 9 c. Site Preparation, Weed Control and Cover Crops…………………….….. 9 d. Planting………………………………………………………………….. 11 e. First Growing Season…………………………………………………… 14 f. Second through Fourth Growing Seasons………………………………. 16 g. -

Salix Från Svalöf Weibull AB
May 2006 Agrobränsle Willow Varieties Tora (Salix schwerinii x S. viminalis). Tora plantations as Inger has a different gene background originates from a cross between a Siberian basket than many of the other varieties willow and the SW-variety Orm. The variety has long shoots and a somewhat lower number of shoots Sherwood (Salix viminalis x S. eriocephala) x than other varieties. Its yield is the highest of all (S. schwerinii x S. viminalis) is a new variety varieties available up to now. Tora is almost free available for planting in 2005. Sherwood is a cross from leaf rust and attacks by gall midges and other between the clone SW 930887, containing genes insects damaging the shoot tips are less common. from S. eriocephala, and the variety Björn, which is Tora is less preferred by game. a male sibling to Tora. Torhild ((Salix schwerinii x S. viminalis) x S. Doris (Salix dasyclados) is a hybrid between the viminalis). Torhild is a cross between the varieties Russian clone SW 901321 and the Polish clone SW Tora and Orm. Torhild has lancet-shaped leaves 881031. Doris is a new variety available for and a straight stem, and few shoots like Tora. The planting in 2005. The variety is very frost tolerant variety has a relatively high yield and a low and resistant to leaf beetles. Doris has low moisture infection of leaf rust. content in harvested wood. The variety has broad leaves and a dense canopy. Sven (Salix viminalis x (S. schwerinii x S. viminalis)). Sven is a cross between the varieties Karin (((Salix schweriini x S. -

Male and Female Plants of Salix Viminalis Perform Similarly to Flooding in Morphology, Anatomy, and Physiology
Article Male and Female Plants of Salix viminalis Perform Similarly to Flooding in Morphology, Anatomy, and Physiology 1, 1, 2 3 3 Fei-fei Zhai y, Hai-dong Li y, Shao-wei Zhang , Zhen-jian Li , Jun-xiang Liu , Yong-qiang Qian 3, Guan-sheng Ju 3, Yun-xing Zhang 1, Long Liu 1, Lei Han 3 and Zhen-yuan Sun 3,* 1 School of Architectural and Artistic Design, Henan Polytechnic University, Century Avenue, Jiaozuo 454000, China; lkyzff@163.com (F.-f.Z.); [email protected] (H.-d.L.); [email protected] (Y.-x.Z.); [email protected] (L.L.) 2 College of Horticulture and Landscape, Henan Vocational College of Agriculture, Zhengzhou 451450, China; [email protected] 3 State Key Laboratory of Tree Genetics and Breeding, Research Institute of Forestry, Chinese Academy of Forestry, Key Laboratory of Tree Breeding and Cultivation, State Forestry Administration, Beijing 10091, China; [email protected] (Z.-j.L.); [email protected] (J.-x.L.); [email protected] (Y.-q.Q.); [email protected] (G.-s.J.); [email protected] (L.H.) * Correspondence: [email protected]; Tel.: +86-010-6288-9626 These authors have contributed equally to this work. y Received: 7 February 2020; Accepted: 11 March 2020; Published: 14 March 2020 Abstract: Salix viminalis L., a dioecious species, is widely distributed in riparian zones, and flooding is one of the most common abiotic stresses that this species suffers. In this study, we investigated the morphological, anatomical, and physiological responses of male vs. female plants of S. viminalis to flooding. The results showed that the plant height and root collar diameter were stimulated by flooding treatment, which corresponded with higher dry weight of the stem and leaf. -

Diversity of Segetal Flora in Salix Viminalis L. Crops Established on Former Arable and Fallow Lands in Central Poland
agriculture Article Diversity of Segetal Flora in Salix viminalis L. Crops Established on Former Arable and Fallow Lands in Central Poland Maria Janicka 1, Aneta Kutkowska 1,* and Jakub Paderewski 2 1 Agronomy Department, Faculty of Agriculture and Biology, Institute of Agriculture, Warsaw University of Life Sciences—SGGW, Nowoursynowska 159, 02-776 Warsaw, Poland; [email protected] 2 Biometry Department, Faculty of Agriculture and Biology, Institute of Agriculture, Warsaw University of Life Sciences—SGGW, Nowoursynowska 159, 02-776 Warsaw, Poland; [email protected] * Correspondence: [email protected] Abstract: The flora of willow (Salix viminalis L.) plantations consists of various plant groups, in- cluding plants related to arable land, called segetal plants. Knowledge of this flora is important for maintaining biodiversity in agroecosystems. The aim of the study was to assess the segetal flora of the willow plantations in central Poland, depending on the land use before the establishment of the plantations (arable land or fallow) and the age of the plantations. Moreover, the aim was also to check for the presence of invasive, medicinal, poisonous and melliferous species. The vegetation accompanying willow was identified based on an analysis of 60 phytosociological relevés performed using the Braun-Blanquet method. For each species, the following parameters were determined: the phytosociological class; family; geographical and historical group; apophyte origin; biological stability; life-form; and status as an invasive, medicinal (herbs), poisonous or melliferous species. The results were statistically processed. Segetal species accounted for 38% of the flora accompanying willow. The plantations on former arable land were richer in segetal species than those on fallow. -

Willows (Salix Spp.) As Pollen and Nectar Sources for Sustaining Fruit and Berry Pollinating Insects
Willows (Salix spp.) as pollen and nectar sources for sustaining fruit and berry pollinating insects D. P. Ostaff1, A. Mosseler1,5, R. C. Johns1, S. Javorek2, J. Klymko3, and J. S. Ascher4 1Natural Resources Canada, Canadian Forest Service Á Atlantic Forestry Centre, P.O. Box 4000, Fredericton, New Brunswick, Canada E3B 5P7; 2Agriculture and Agri-Food Canada, Atlantic Food Horticulture Research Centre, 32 Main Street, Kentville, Nova Scotia, Canada B4N 1J5 ([email protected]); 3Atlantic Canada Conservation Data Centre, P.O. Box 6416, Sackville, New Brunswick, Canada E4L 1G6; and 4Department of Biological Sciences, National University of Singapore, 14 Science Drive 4, Singapore 117543. Received 17 September 2014, accepted 22 January 2015. Published on the web 23 January 2015. Ostaff, D. P., Mosseler, A., Johns, R. C., Javorek, S., Klymko, J. and Ascher, J. S. 2015. Willows (Salix spp.) as pollen and nectar sources for sustaining fruit and berry pollinating insects. Can. J. Plant Sci. 95: 505Á516. Willows (Salix spp.) are ubiquitous in the northern hemisphere, serving as an important pollen and nectar resource for insect pollinators and for the enhancement of insect-pollinated agricultural crops such as fruits and berries. We used a common-garden field test containing seven native North American willow species to assess attractiveness of male and female flower catkins by documenting visits of Andrena spp. (Apoidea: Anthophila), other wild bees (all native), and flower flies (Syrphidae). Most willows in Canada’s Maritimes begin flowering very early in spring, as the first wild pollinators become active following winter, and stop flowering by mid-May. -

Growth, Physiology, and Phytoextraction Potential of Poplar and Willow Established in Soils Amended with Heavy-Metal Contaminate
Journal of Environmental Management 239 (2019) 352–365 Contents lists available at ScienceDirect Journal of Environmental Management journal homepage: www.elsevier.com/locate/jenvman Research article Growth, physiology, and phytoextraction potential of poplar and willow T established in soils amended with heavy-metal contaminated, dredged river sediments ∗ Andrej Pilipovića, Ronald S. Zalesny Jr.b, , Srđan Rončevićc, Nataša Nikolićd, Saša Orlovića, Jelena Beljinc, Marina Katanića a Institute of Lowland Forestry and Environment, University of Novi Sad, Novi Sad, Serbia b Institute for Applied Ecosystem Studies, Northern Research Station, USDA Forest Service, Rhinelander, WI, USA c Faculty of Sciences, Department of Chemistry, Biochemistry and Environmental Protection, University of Novi Sad, Novi Sad, Serbia d Faculty of Sciences, Department of Biology and Ecology, University of Novi Sad, Novi Sad, Serbia ARTICLE INFO ABSTRACT Keywords: Phytotechnologies have been used worldwide to remediate and restore damaged ecosystems, especially those Bioconcentration factor caused by industrial byproducts leaching into rivers and other waterways. The objective of this study was to test Biomass the growth, physiology, and phytoextraction potential of poplar and willow established in soils amended with Phytoremediation heavy-metal contaminated, dredged river sediments from the Great Bačka Canal near Vrbas City, Serbia. The Populus deltoides Bartr. ex Marsh sediments were applied to greenhouse-grown trees of Populus deltoides Bartr. ex Marsh. clone ‘Bora’ and Salix Salix viminalis L. viminalis L. clone ‘SV068’. Individual pots with trees previously grown for two months were amended with 0, 0.5 Tree uptake and 1.0 kg of sediment containing 400 mg Cr kg−1, 295 mg Cu kg−1, 465 mg Zn kg−1, 124 mg Ni kg−1, 1.87 mg Cd kg−1, and 61 mg Pb kg−1. -

Salix: a Viable Option for Phytoremediation
African Journal of Environmental Science and Technology Vol. 5(8), pp. 567-571, August, 2011 Available online at http://www.academicjournals.org/AJEST ISSN 1996-0786 ©2011 Academic Journals Review Salix: A viable option for phytoremediation Bilal Ahmad Wani*, Amina Khan and R. H. Bodha Wood Science Laboratory, Department of Botany, University of Kashmir, Hazratbal, Srinagar –190006, India. Accepted 21 June, 2011 Economic importance of Salix is currently increasing and emerging in a wide array of practical applications to restore damaged ecosystems. Salix spp which are characterized by particular physiological adaptations and ecological resilience are predisposed to use in conservation and environmental projects in many climatic zones and adverse micro site conditions. The present paper throw light on the current use of willows well beyond wetland and riparian situations such as in phytoremedation (phytoextraction, phytodegradation, rhizofiltration and phytostabilization) based on literature review. Key words: Salix, phytoremedation, phytoextraction, phytodegradation, rhizofiltration, phytostabilization, ecosystem. INTRODUCTION Stupendous increase in population over the years herbivores from aphids to caterpillars feed on willows, exceeding one billion, which is about 1/6th of the world and support a large food-web of higher trophic level population in our country India, exerts heavy pressure on organisms. Many animals depend on willows for food our renewable and non renewable natural resources. (mostly leaf, stem and bud tissue) and shelter; willows This increasing pressure is responsible for huge provide browse for large number of animals and willow depletion of these resources resulting not only in wood is a preferred food and building material for alarming deficit between demand and supply of forests beavers (Smith et al., 1978).