Salix: a Viable Option for Phytoremediation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
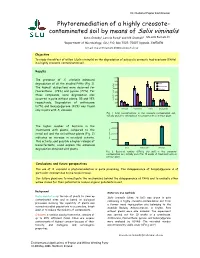
Phytoremediation of a Highly Creosote- Contaminated Soil by Means of Salix Viminalis
International Poplar Commission Phytoremediation of a highly creosote- contaminated soil by means of Salix viminalis Karin Önneby1, Leticia Pizzul1 and Ulf Granhall1 Mauritz Ramstedt 1Department of Microbiology, SLU, P.O. Box 7025, 75007 Uppsala, SWEDEN Email:E-mail: mauritz.ramstedt@m [email protected] Objective To study the effect of willow (Salix viminalis) on the degradation of polycyclic aromatic hydrocarbons (PAHs) in a highly creosote-contaminated soil. Results 1600 The presence of S. viminalis enhanced 1400 Initial degradation of all the studied PAHs (Fig. 1). 1200 The highest dissipations were observed for Without plant 1000 With plant fluoranthene (79%) and pyrene (77%). For 800 those compounds, some degradation also 600 occurred in pots without plants, 35 and 19% 400 Concentration (mg/kg dw) Concentration respectively. Degradation of anthracene 200 (67%) and benzo[a]pyrene (43%) was found 0 Anthracene Fluoranthene Pyrene Benzo[a]pyrene only in pots with S. viminalis. Fig. 1. PAH concentrations in the creosote-contaminated soil, initially and after 10 weeks of treatment with or without plant. 4000000 The higher number of bacteria in the 3000000 treatments with plants, compared to the initial soil and the soil without plants (Fig. 2) 2000000 indicates an increase in microbial activity. CFU (dw) /g soil 1000000 This activity, and possibly a higher release of biosurfactants, could explain the enhanced 0 degradation obtained with plants. Initial Without plant With plant Fig. 2. Bacterial number (CFU/g dry soil) in the creosote- contaminated soil, initially and after 10 weeks of treatment with or without plant. Conclusions and future perspectives The use of S. -

Short Rotation Intensive Culture of Willow, Spent Mushroom Substrate
plants Article Short Rotation Intensive Culture of Willow, Spent Mushroom Substrate and Ramial Chipped Wood for Bioremediation of a Contaminated Site Used for Land Farming Activities of a Former Petrochemical Plant Maxime Fortin Faubert 1 , Mohamed Hijri 1,2 and Michel Labrecque 1,* 1 Institut de Recherche en biologie végétale, Université de Montréal and Jardin Botanique de Montréal, 4101 Sherbrooke East, Montréal, QC H1X 2B2, Canada; [email protected] (M.F.F.); [email protected] (M.H.) 2 African Genome Center, Mohammed VI Polytechnic University (UM6P), Lot 660, Hay Moulay Rachid, Ben Guerir 43150, Morocco * Correspondence: [email protected]; Tel.: +1-514-978-1862 Abstract: The aim of this study was to investigate the bioremediation impacts of willows grown in short rotation intensive culture (SRIC) and supplemented or not with spent mushroom substrate (SMS) and ramial chipped wood (RCW). Results did not show that SMS significantly improved either biomass production or phytoremediation efficiency. After the three growing seasons, RCW- amended S. miyabeana accumulated significantly more Zn in the shoots, and greater increases of some PAHs were found in the soil of RCW-amended plots than in the soil of the two other ground Citation: Fortin Faubert, M.; Hijri, cover treatments’ plots. Significantly higher Cd concentrations were found in the shoots of cultivar M.; Labrecque, M. Short Rotation ‘SX61’. The results suggest that ‘SX61’ have reduced the natural attenuation of C10-C50 that occurred Intensive Culture of Willow, Spent in the unvegetated control plots. The presence of willows also tended to increase the total soil Mushroom Substrate and Ramial concentrations of PCBs. -

The Use of Vegetation in the Stabilization, Reclamation, and Remediation of Impacted INDOT Soils
Final Report FHWA/IN/JTRP-2004/17 The Use of Vegetation in the Stabilization, Reclamation, and Remediation of Impacted INDOT Soils by Mark S. McClain Graduate Research Assistant and M. Katherine Banks Professor School of Civil Engineering and A. Paul Schwab Department of Agronomy Purdue University Joint Transportation Research Program Project No. C-36-68T File No. 4-7-20 SPR-2624 Prepared in Cooperation with the Indiana Department of Transportation and the U.S. Department of Transportation Federal Highway Administration The contents of this report reflect the views of the author who is responsible for the facts and the accuracy of the data presented herein. The contents do not necessarily reflect the official views or policies of the Indiana Department of Transportation or the Fedeeral Highway Administration at the time of publication. This report does not constitute a standard, specification, or regulation. Purdue University West Lafayette, Indiana 47907 October 2004 TECHNICAL Summary Technology Transfer and Project Implementation Information INDOT Research TRB Subject Code: 23-8 Ecological Abatement October 2004 Publication No.: FHWA/IN/JTRP-2004/17, SPR-2624 Final Report The Use of Vegetation in the Stabilization, Reclamation, and Remediation of Impacted INDOT Soils Introduction Soils can be severely impacted by transportation- Phytoremediation uses plants to degrade, extract, related activities including highway construction, contain, or immobilize contaminants from soil and renovations, maintenance, and accidental spills. water. Phytoremediation is an innovative, cost- Reclamation or remediation of soils contaminated effective alternative to more conventional treatment by salts, solvents, paints, petroleum, and metals methods used in the remediation of hazardous may be necessary to comply with current waste sites. -

Phytoremediation Potential of Fast-Growing Energy Plants: Challenges and Perspectives – a Review
Pol. J. Environ. Stud. Vol. 29, No. 1 (2020), 505-516 DOI: 10.15244/pjoes/101621 ONLINE PUBLICATION DATE: 2019-09-10 Review Phytoremediation Potential of Fast-Growing Energy Plants: Challenges and Perspectives – a Review Martin Hauptvogl1*, Marián Kotrla2, Martin Prčík1, Žaneta Pauková3, Marián Kováčik4, Tomáš Lošák5 1Department of Environmental Management, Slovak University of Agriculture in Nitra, Slovakia 2Department of Regional Bioenergetics, Slovak University of Agriculture in Nitra, Slovakia 3Department of Regional Bioenergetics , Slovak University of Agriculture in Nitra, Slovakia 4Department of EU Policies, Slovak University of Agriculture in Nitra, Slovakia 5Department of Environmentalistics and Natural Resources, Mendel University in Brno, Czech Republic Received: 23 July 2018 Accepted: 15 December 2018 Abstract Contamination of soil by toxic elements is a global issue of growing importance due to the increased anthropogenic impact on the natural environment. Conventional methods of soil decontamination possess disadvantages in forms of environmental and financial burdens. This fact leads to the search for alternative approaches of remediation of contaminated sites. One such approach includes phytoremediation. Phytoremediation advantages consist of low costs and small environmental impact. Several fast-growing energy plant species are suitable for phytoremediation purposes. Our article focuses on the phytoremediation potential of energy woody crops of Salix and Populus, and energy grasses Miscanthus and Arundo, which are grown primarily for biomass production. This approach links the environmentally friendly and economically less demanding remediation approach with the production of the local sustainable form of energy that decreases dependency on external energy supplies. Energy plants are able to provide high biomass yields in a short period of time, they are resistant against abiotic stress conditions and have the ability to accumulate toxic substances, thus helping to restore the desirable soil properties. -

Interest Increases in Using Plants for Environmental Remediation
Specialty Plant Products Interest Increases in Using Plants For Environmental Remediation In an effort to meet environmental regulations of the last three decades, environmental remediation has developed into a multibillion dollar industry. The high cost of many traditional methods is causing many organizations to look to lower cost alternatives. Bioremediation is a commercial remediation technology with a growing market and continuing research. Phytoremediation is another potential low-cost technology that is currently being investigated for many remediation applications. Health and environmental risks of pollution have become Table 14--Soil remediation technologies more apparent throughout the world over the past several Method In situ Ex situ decades. Air, water, and soil contaminants can include nu- merous organic and inorganic substances, such as munici- Physical Soil vapor extraction Landfilling pal waste and sewage, various gaseous emissions, fertiliz- Thermally enhanced Incineration ers, pesticides, chemicals, heavy metals, and radionuclides soil vapor extraction Thermal desorption (radioactive substances). Contaminants can cause land and Containment systems Soil vapor extraction groundwater to be unusable. In addition, animals and in- and barriers sects may come in contact with a contaminant, thus intro- ducing a toxic substance into the food chain. Because of in- Chemical Soil flushing Soil washing creased public awareness and concern, environmental Solidification Solidification regulations have been created to not only prevent -

Cultivated Willows Would Not Be Appropriate Without Mention of the ‘WEEPING WILLOW’
Alexander Robertson Notes on willows cultivated in Scotland and a few sketches of willows sampled from my clonal collection HISTORICAL NOTES Since the knowledge of willows is of great antiquity, it is with the ancient Greeks and Romans we shall begin, for among these people numerous written records remain. The growth habit, ecology, cultivation and utilization of willows was well— understood by Theophrastus, Ovid, Herodotus, Pliny and Dioscorides. Virgil was also quite familiar with willow, e.g. Damoetas complains that: “Galatea, saucy girl, pelts me with apples and then runs off to the willows”. ECLOGIJE III and of foraging bees: “Far and wide they feed on arbutus, pale-green willows, on cassia and ruddy crocus .. .“ GEORGICS IV Theophrastus of Eresos (370—285 B.C.) discussed many aspects of willows throughout his Enquiry into Plants including habitats, wood quality, coppicing and a variety of uses. Willows, according to Theophrastus are lovers of wet places and marshes. But he also notes certain amphibious traits of willows growing in mountains and plains. To Theophrastus they appeared to possess no fruits and quite adequately reproduced themselves from roots, were tolerant to flooding and frequent coppicing. “Even willows grow old and when they are cut, no matter at what height, they shoot up again.” He described the wood as cold, tough, light and resilient—qualities which made it useful for a variety of purposes, especially shields. Such were the diverse virtues of willow that he suggested introducing it for plant husbandry. Theophrastus noted there were many different kinds of willows; three of the best known being black willow (Salix fragilis), white willow (S. -

Phytoremediation of Wood Preservatives FWRC
Phytoremediation of Wood Preservatives FWRC Soil and waste contaminated with wood for contaminated areas: 1) enhanced microbial preservatives have been found in many former degradation of contaminants within the rhizosphere, and active wood treating plants, resulting from 2) hyperaccumulators where plants uptake and store past practices and accidental spillage. During the harmful contaminants, commonly heavy metals, in spring of 2001, newspaper articles in Florida raised their roots and shoots, 3) rhizofiltration where plant a public alarm over the perception that arsenate roots absorb, concentrate, or precipitate heavy metal from CCA (chromated copper arsenate) treated ions from water and, 4) phytovolatilization where wood—used in playground equipment— was leaching plants uptake volatile organic compounds (VOCs) in into soils and potentially into groundwater reserves. groundwater allowing them to be released into the As a result, CCA treated wood was voluntarily atmosphere via the stomata openings. taken off the market by the industry. Physical Surface waters and shallow aquifers were the removal of contaminated soils followed by treatment first sites where plants were applied as a method of technologies—soil washing, solidification cleanup. Many different plants and trees can and stabilization, chemical remove or degrade toxic pollutants. treatment,vitrification, thermal The primary disadvantage in using desorption, electrokinetics, trees for environmental cleanup and incineration—is not a is the time needed for tree cost effective soil cleanup growth. Faster growing method for heavy metal flora like grasses may be contaminated water. The suited for some situations. most common technologies Although the roots of include: coagulation/ grasses do not penetrate filtration (activated carbon), the soil at the same depths lime softening, chemical as tree roots, they can treatment, reverse osmosis, proliferate within the topsoil. -

Sustainable Use of Plants for Heavy Metal Removal from Water: Phytoremediation
A.P. Bhat and P.P. Bhat (2016) Int J Appl Sci Biotechnol, Vol 4(2): 150-154 DOI: 10.3126/ijasbt.v4i2.14742 Mini Review SUSTAINABLE USE OF PLANTS FOR HEAVY METAL REMOVAL FROM WATER: PHYTOREMEDIATION Akash P. Bhat1* and Pooja P. Bhat2 1Department of Chemical Engineering, Thadomal Shahani Engineering College, Bandra West, Mumbai 400050, Maharashtra, India, 2Department of Botany, Ramnarain Ruia College, Matunga, Mumbai 400019, Maharashtra, India. *Corresponding Author email: [email protected] Abstract There are various methods for removal of heavy metals from contaminated water and many of them can be costly and also consume a lot of resources. Phytoremediation is the use of plants as a filter for removal of unwanted elements and substances from contaminated water. This process is called rhizofiltration. Phytoremediation has not achieved a lot of importance on large scale level. This review- study shows how several species like Brassica juncea and Chenopodium amaranticolor, Pistia stratiotes, Helianthus annuus L. and Phaseolus vulgaris L. var. vulgaris, Eleocharis acicularis, Lemna minor L., Phragmites australis and Eichhornia Crassipes can be used for effective removal of heavy metals. These species are selected based on a review on various studies on rhizofiltration. Hence rhizofiltration can be an eco-friendly and innovative method of removal of heavy metals and has to be applied for large scale treatment of heavy metals in real time waters. Keywords: Phytoremediation; Rhizofiltration; Plants; Heavy metals; Removal Introduction processes should be at the utmost important place for Heavy metals and pollutants in water and aqueous wastes combating global warming and pollution. are a huge threat currently to the environment. -

List of Publications —MARIA GREGER
List of Publications —MARIA GREGER Scientific Publications (Paper with review system = R; Included in thesis = X) 1. RX Greger M. & Lindberg S., 1986. Effects of Cd2+ and EDTA on young sugar beets (Beta vulgaris). I. Cd2+uptake and sugar accumulation. — Physiologia Plantarum 66: 69-74. 2. RX Greger M. & Lindberg S., 1987. Effects of Cd2+ and EDTA on young sugar beets (Beta vulgaris). II. Net uptake and distribution of Mg2+, Ca2+ and Fe2+/Fe3+. — Physiologia Plantarum 69: 81-86. 3. RX Greger M., 1989. Cadmium Effects on Carbohydrate Metabolism in Sugar Beet (Beta vulgaris). — Thesis, Stockholm University, ISBN 91-7146-717-3 4. R Kronestedt-Robards E. C., Greger M. & Robards A. W., 1989. The nectar of the Strelizia Reginæ flower. — Physiologia Plantarum 77: 341-346. 5. R X Greger M., Brammer E. S., Lindberg S., Larsson G. & Idestam-Almquist J., 1991. Uptake and physiological effects of cadmium in sugar beets (Beta vulgaris) related to mineral provision. — Journal of Experimental Botany 42: 729-737. 6. R Lindberg S., Szynkier K. & Greger M., 1991. Aluminum effects on transmembrane potential in cells of fibrous roots of sugar beet. — Physiologia Plantarum 83: 54-62. 7. R X Greger M. & Ögren E., 1991. Direct and indirect effects of Cd2+ on the photosynthesis and CO2-assimilation in sugar beets (Beta vulgaris). — Physiologia Plantarum 83: 129-135. 8. R Greger M. & Kautsky L., 1991. Effects of Cu, Pb, and Zn on two species of Potamogeton grown in field conditions. — Vegetatio 97: 173-184. 9. R X Greger M. & Bertell G., 1992. Effects of Ca2+ and Cd2+ on the carbohydrate metabolism in sugar beet (Beta vulgaris). -

THE ABIOTIC STRESS RESPONSE of HYDROPONIC VETIVER GRASS (Chrysopogon Zizanioides L.) to ACID MINE DRAINAGE and ITS POTENTIAL for ENVIRONMENTAL REMEDIATION
Michigan Technological University Digital Commons @ Michigan Tech Dissertations, Master's Theses and Master's Reports 2018 THE ABIOTIC STRESS RESPONSE OF HYDROPONIC VETIVER GRASS (Chrysopogon zizanioides L.) TO ACID MINE DRAINAGE AND ITS POTENTIAL FOR ENVIRONMENTAL REMEDIATION Jef Kiiskila Michigan Technological University, [email protected] Copyright 2018 Jef Kiiskila Recommended Citation Kiiskila, Jef, "THE ABIOTIC STRESS RESPONSE OF HYDROPONIC VETIVER GRASS (Chrysopogon zizanioides L.) TO ACID MINE DRAINAGE AND ITS POTENTIAL FOR ENVIRONMENTAL REMEDIATION", Open Access Dissertation, Michigan Technological University, 2018. https://doi.org/10.37099/mtu.dc.etdr/763 Follow this and additional works at: https://digitalcommons.mtu.edu/etdr Part of the Biochemistry Commons, Environmental Sciences Commons, and the Plant Biology Commons THE ABIOTIC STRESS RESPONSE OF HYDROPONIC VETIVER GRASS (Chrysopogon zizanioides L.) TO ACID MINE DRAINAGE AND ITS POTENTIAL FOR ENVIRONMENTAL REMEDIATION By Jeffrey D. Kiiskila A DISSERTATION Submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY In Biochemistry and Molecular Biology MICHIGAN TECHNOLOGICAL UNIVERSITY 2018 © 2018 Jeffrey D. Kiiskila This dissertation has been approved in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY in Biochemistry and Molecular Biology. Department of Biological Sciences Dissertation Advisor: Rupali Datta Committee Member: Dibyendu Sarkar Committee Member: Tarun K. Dam Committee Member: Victor Busov Department -

Phytoextraction of Cadmium: the Advantages, Limitations, Cost and Potential Improvements of This Remediation Technique
Phytoextraction of Cadmium: the advantages, limitations, cost and potential improvements of this remediation technique. Becky Meyerholt Abstract Throughout the United States there are multiple sites where the soil has been contaminated with heavy metals such as zinc, cadmium, lead, and arsenic. Due of the risk that these metals pose to humans and the local ecosystem, these contaminated sites are not fit for food production until the contamination is remediated. Because of the cost and perceived invasiveness of traditional ex-situ remediation techniques, there has been an increase in the number of sites that are remediated using in-situ techniques such as phytoremediation. Phytoremediation uses plants to reduce the concentration of a contaminant and/or reduce the risk of exposure from a contaminant in the environment. Phytoextraction uses plants to remove a soil contaminate by sequestering it in the plant tissues, which are then harvested and removed. As with all remediation techniques, phytoextraction has a limited effectiveness. Its two main limitations are: metal toxicity to plants at high concentrations and the cost to dispose of the plant tissues. Recent studies have proposed different methods to overcome these limitations through the use of genetic engineering and coupling the disposal of harvested biomass with the production of energy, biofuels or as a source for the mining of valuable metals. 1. Introduction: The land area of our planet is finite and as the world population grows, it is important that degraded lands are restored for productive uses such as food and sustainable energy production. Land degradation, or the loss of productive value from a given area of land, can occur due to multiple reasons including chemical contamination from both natural and anthropogenic sources. -

Phytoremediation of Metal Contamination Using Salix (Willows)
University of Denver Digital Commons @ DU Electronic Theses and Dissertations Graduate Studies 1-1-2015 Phytoremediation of Metal Contamination Using Salix (Willows) Gordon J. Kersten University of Denver Follow this and additional works at: https://digitalcommons.du.edu/etd Part of the Ecology and Evolutionary Biology Commons, and the Other Environmental Sciences Commons Recommended Citation Kersten, Gordon J., "Phytoremediation of Metal Contamination Using Salix (Willows)" (2015). Electronic Theses and Dissertations. 1034. https://digitalcommons.du.edu/etd/1034 This Thesis is brought to you for free and open access by the Graduate Studies at Digital Commons @ DU. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ DU. For more information, please contact [email protected],[email protected]. Phytoremediation of Metal Contamination using Salix (willows) ______________________ A Thesis Presented to The Faculty of Natural Sciences and Mathematics University of Denver ______________________ In Partial Fulfillment Of the Requirements for the Degree Master of Science ______________________ By Gordon J. Kersten August 2015 Advisor: Martin F. Quigley Author: Gordon J. Kersten Title: Phytoremediation of Metal Contamination using Salix (willows) Advisor: Martin F. Quigley Degree Date: August 2015 ABSTRACT Abandoned hardrock mines and the resulting Acid Mine Drainage (AMD) are a source of vast, environmental degradation that are toxic threats to plants, animals, and humans. Cadmium (Cd) and lead (Pb) are metal contaminants often found in AMD. In my mine outwash water samples, cadmium and lead concentrations were 19 and 160 times greater than concentrations in control waterways, and 300 and 40 times greater than EPA Aquatic Life Use water quality standards, respectively.