Predicting the Impacts of Climate Change on the Distribution and Conservation of Endemic Forest Land Snails of Madeira Island
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SPIRULA - Speciale Uitgave, Supplement Nr
SPIRULA - Speciale uitgave, supplement nr. 2 13 Eponiemen betreffende personen verbonden aan de NMV Gijs+C. Kronenberg Inleiding In het kader van het 50-jarig jubileum van de NMV werd een lijst gepubliceerd (KUIPER, 1984: 1589-1590) met In eponiemen, dat wil zeggen, weekdiersoortenvernoemd naar Nederlandse malacologen. latere jaren verschenen hierop een aantal aanvullingen (KUIPER, 1986; KUIPER, 1988; KUIPER, 1989; KUIPER, 1991; KUIPER, 1992; KUIPER, 1993; KUIPER 1995). Na 1995 zijn er verder geen overzichten meer samengesteld. Helaas is aan het initiatiefvan met KUIPER (opera cit.) geen vervolg gegeven, zodat we een achterstand zitten, maar die nu wordt weggewerkt middels deze vernieuwde lijst. Criteria perd door [KRONENBERG] (2001). Om in de lijst opgenomen te worden dientte worden voldaan Daarnaast hebben deze criteriaook als gevolg dat de namen aan drie criteria: Trochus wilsi PICKERY, 1989 (KUIPER 1992); Ischnochiton Men moet lid zijn (geweest) van de N.M.V. ofeen plaatselij- vanbellei KAAS; en Notoplax richardi KAAS [ook vernoemd ke schelpenwerkgroep enigszins geliëerd aan de NMV, of naar de heer R.A. VAN BELLE] (KUIPER, 1993) uitde lijst zijn daar in zeer nauwe (privé) relatie mee staan (huwelijk, geschrapt daar deze personen niet de Nederlandse nationali- dank samenwonen, kinderen) als voor de vele jaren trouwe teit (gehad) hebben, en taxa vernoemd naar Prof. Dr. J.K.L. Of in niet daar deze ondersteuning en dergelijke. men moet dusdanige pro- MARTIN opgenomen zijn niet de Nederlandse fessioneel malacologische relatie hebben gestaan dat de nationaliteitheeft gehad. auteur het taxon dat tot wilde in het Taxa beschreven door b.v. uit van uiting brengen ver- E.J. -

Spatial Predictive Distribution Modelling of Madeira's Endemic
DEPARTAMENTO DE ZOOLOGIA FACULDADE DE CIÊNCIAS E TECNOLOGIA UNIVERSIDADE DE COIMBRA Spatial predictive distribution modelling of Madeira’s endemic land snail species Dinarte Nuno Freitas Teixeira 2009 REGIÃO AUTÓNOMA DA REPÚBLICA PORTUGUESA UNIÃO EUROPEIA MADEIRA FSE DEPARTAMENTO DE ZOOLOGIA FACULDADE DE CIÊNCIAS E TECNOLOGIA UNIVERSIDADE DE COIMBRA Spatial predictive distribution modelling of Madeira’s endemic land snail species Dissertação apresentada à Universidade de Coimbra para cumprimento dos requisitos necessários à obtenção do grau de Mestre em Ecologia, realizada sob a orientação científica do Professor Doutor José Paulo Sousa (Universidade de Coimbra) e do Professor Doutor José Manuel Jesus (Universidade da Madeira). Dinarte Nuno Freitas Teixeir a 2009 O presente trabalho foi financiado pelo Centro de Ciência e Tecnologia da Madeira (CITMA), através da bolsa de Mestrado FSE BM I/2008 – 531, ao abrigo do Programa Operacional de Valorização do Potencial Humano e Coesão Social da RAM (RUMOS). REGIÃO AUTÓNOMA DA MADEIRA REPÚBLICA PORTUGUESA UNIÃO EUROPEIA FSE À Susana AGRADECIMENTOS Esta tese é o resultado de um trabalho conjunto para o qual muitos contribuíram e aos quais desejo reconhecer e deixar o meu agradecimento. Ao professor Doutor José Paulo Sousa, meu orientador, pela indispensável ajuda, paciência e orientação científica. Ao professor Doutor José Manuel Jesus, meu orientador, pela amizade e apoio desde os primeiros momentos. Pelo seu empenho, conselhos transmitidos, chamadas à razão e orientação científica o meu muito obrigado. Ao Doutor Pedro Cardoso, meu orientador e a quem muito devo, pelo constante acompanhamento e disponibilidade, amizade e orientação científica. Por tudo o que me ensinou, pela motivação e animo que sempre me transmitiu, e, acima de tudo, pela manutenção da objectividade do trabalho. -

Diplom-Biologe KLAUS GROH Malakozoologe Und Naturschützer – 65 Jahre
53 Mitt. dtsch. malakozool. Ges. 94 53 – 70 Frankfurt a. M., November 2015 Diplom-Biologe KLAUS GROH Malakozoologe und Naturschützer – 65 Jahre CARSTEN RENKER & JÜRGEN H. JUNGBLUTH th Abstract: The 65 birthday of KLAUS GROH is a good occasion to give a retrospect of his life and hitherto existing achievement. Beside his vita we summarize his malacological work, give an overview about the projects for the protection of species, have a look on his tremendous impetus for the worldwide distribution of malacological knowledge by the establishment of the CHRISTA HEMMEN-Verlag, later ConchBooks, as publishing house, book trader and antiquarian. Last but not least we give a summary of his scientific achievements culminating in 206 publications and containing descriptions of up to now 42 specific taxa. Keywords: KLAUS GROH, biography, bibliography, malacology, freshwater mussels, Hesse, Rhineland- Palatinate, Luxembourg Zusammenfassung: Der 65. Geburtstag von KLAUS GROH wird zum Anlass genommen einen Rückblick auf sein bisheriges Leben und Wirken zu geben. Neben der Vita werden vor allem seine malakologische Arbeit und sein ehrenamtliches Engagement in zahlreichen malakologischen Verbänden und Naturschutzvereinen betrachtet. KLAUS GROH nahm außerdem einen enormen Einfluss auf die weltweite Verbreitung malako- logischen Wissens durch die Gründung des CHRISTA HEMMEN-Verlags, später ConchBooks, als Verlagshaus, Buchhandlung und Antiquariat. Schließlich gilt es seine wissenschaftlichen Verdienste zu würdigen, die in 206 Publikationen und Neubeschreibungen 42 spezifischer Taxa kulminieren. Vita Schulzeit Am 22. Mai 1949 wurde KLAUS GROH in Darmstadt als Sohn des Bauschlossers HELMUT GROH und seiner Ehefrau ANNELIESE, geb. FEDERLEIN geboren. Er besuchte die Volksschulen in Langen/Hessen und Kirchheim unter Teck/Baden-Württemberg (1955-1959), es folgte der Besuch der Realschule in Langen/Hessen (1959-1965), dort schloss er auch seine Schulzeit mit der „Mittleren Reife“ ab. -

Amaia Caro Aramendia
The genus Pyrenaearia (Gastropoda, Helicoidea): Molecular and Morphological Systematics, Biogeography and Population Dynamics Pyrenaearia generoa (Gastropoda, Helicoidea): Sistematika Molekularra eta Morfologikoa, Biogeografia eta Populazio Dinamika PhD thesis Vitoria-Gasteiz, 2019 Amaia Caro Aramendia The genus Pyrenaearia (Gastropoda, Helicoidea): Molecular and Morphological Systematics, Biogeography and Population Dynamics Pyrenaearia generoa (Gastropoda, Helicoidea): Sistematika Molekularra eta Morfologikoa, Biogeografia eta Populazio Dinamika A thesis submitted by Amaia Caro Aramendia for the degree of Doctor of Philosophy, under the supervision of Dr. Benjamín Juan Gómez-Moliner and Dr. María José Madeira University of the Basque Country, Vitoria-Gasteiz, 2019 Zoologia eta Animalia Biologia Zelulen Saila Dpto. Zoología y Biología Celular Animal (cc)2019 AMAIA CARO ARAMENDIA (cc by-nc-nd 4.0) Astiro igo, barraskilotxo Fuji mendia da hau! Kobayashi Issa-ren haikua To the little things that run the world Esker onak Acknowledgements Tesi bat ez da pertsona bakar batena, bidean zehar laguntzen duten pertsona guztiei esker sortutako lana da eta, beraz, lehen orriek haien laguntza eskertzeko izan behar dute: En primer lugar me gustaría agradecer a mis directores, Benjamín Gómez-Moliner y María José Madeira. A Benjamín, por darme la oportunidad de entrar en el grupo de investigación y confiar en que podría realizar esta tesis. Gracias por compartir tus extensos conocimientos y por descubrirme el mundo de la malacología, que sin duda no habría encontrado por mi cuenta y ha resultado de lo más interesante. A Marijo, porque desde el principio y hasta el final has estado siempre ahí para guiarme, animarme y para ayudarme en todo lo que hiciese falta pero, sobre todo, por mostrarme que es posible compaginar este trabajo con una vida fuera de él. -

(Approx) Mixed Micro Shells (22G Bags) Philippines € 10,00 £8,64 $11,69 Each 22G Bag Provides Hours of Fun; Some Interesting Foraminifera Also Included
Special Price £ US$ Family Genus, species Country Quality Size Remarks w/o Photo Date added Category characteristic (€) (approx) (approx) Mixed micro shells (22g bags) Philippines € 10,00 £8,64 $11,69 Each 22g bag provides hours of fun; some interesting Foraminifera also included. 17/06/21 Mixed micro shells Ischnochitonidae Callistochiton pulchrior Panama F+++ 89mm € 1,80 £1,55 $2,10 21/12/16 Polyplacophora Ischnochitonidae Chaetopleura lurida Panama F+++ 2022mm € 3,00 £2,59 $3,51 Hairy girdles, beautifully preserved. Web 24/12/16 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 30mm+ € 4,00 £3,45 $4,68 30/04/21 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 27.9mm € 2,80 £2,42 $3,27 30/04/21 Polyplacophora Ischnochitonidae Stenoplax limaciformis Panama F+++ 16mm+ € 6,50 £5,61 $7,60 Uncommon. 24/12/16 Polyplacophora Chitonidae Acanthopleura gemmata Philippines F+++ 25mm+ € 2,50 £2,16 $2,92 Hairy margins, beautifully preserved. 04/08/17 Polyplacophora Chitonidae Acanthopleura gemmata Australia F+++ 25mm+ € 2,60 £2,25 $3,04 02/06/18 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 41mm+ € 4,00 £3,45 $4,68 West Indian 'fuzzy' chiton. Web 24/12/16 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 32mm+ € 3,00 £2,59 $3,51 West Indian 'fuzzy' chiton. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 44mm+ € 5,00 £4,32 $5,85 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F++ 35mm € 2,50 £2,16 $2,92 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 29mm+ € 3,00 £2,59 $3,51 Caribbean. -

Malacologica
FOLIA Folia Malacol. 24(3): 111–177 MALACOLOGICA ISSN 1506-7629 The Association of Polish Malacologists Faculty of Biology, Adam Mickiewicz University Bogucki Wydawnictwo Naukowe Poznań, September 2016 http://dx.doi.org/10.12657/folmal.024.008 PATTERNS OF SPATIO-TEMPORAL VARIATION IN LAND SNAILS: A MULTI-SCALE APPROACH SERGEY S. KRAMARENKO Mykolaiv National Agrarian University, Paryzka Komuna St. 9, Mykolaiv, 54020, Ukraine (e-mail: [email protected]) ABSTRACT: Mechanisms which govern patterns of intra-specific vatiation in land snails were traced within areas of different size, using Brephulopsis cylindrica (Menke), Chondrula tridens (O. F. Müller), Xeropicta derbentina (Krynicki), X. krynickii (Krynicki), Cepaea vindobonensis (Férussac) and Helix albescens Rossmässler as examples. Morphometric shell variation, colour and banding pattern polymorphism as well as genetic polymorphism (allozymes and RAPD markers) were studied. The results and literature data were analysed in an attempt to link patterns to processes, with the following conclusions. Formation of patterns of intra- specific variation (initial processes of microevolution) takes different course at three different spatial scales. At micro-geographical scale the dominant role is played by eco-demographic characteristics of the species in the context of fluctuating environmental factors. At meso-geographical scale a special part is played by stochastic population-genetic processes. At macro-geographical scale more or less distinct clinal patterns are associated with basic macroclimatic -
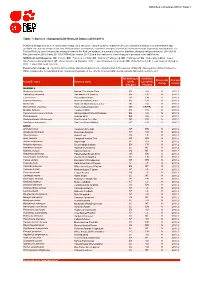
Table 7: Species Changing IUCN Red List Status (2010-2011)
IUCN Red List version 2011.2: Table 7 Table 7: Species changing IUCN Red List Status (2010-2011) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2010 (IUCN Red List version 2010.4) and 2011 (IUCN Red List version 2011.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2010) List (2011) change version Category Category MAMMALS Bradypus torquatus Maned Three-toed Sloth EN VU N 2011.1 Callicebus oenanthe San Martin Titi Monkey EN CR N 2011.1 Equus ferus Przewalski's Horse CR EN G 2011.2 -

CITES in Nederland
Toelichting op de wettelijke maatregelen ter uitvoering van CITES in Nederland MINISTERIE VAN LANDBOUW, NATUURBEHEER EN VISSERIJ 1995 79x908(x2) TOELICHTING OP DE WETTELIJKE MAATREGELEN TER UITVOERING VAN CITES IN NEDERLAND o f h o e k Ministerie van Landbouw, Natuurbeheer en Visserij Directie Natuurbeheer CITES-bureau Postbus 20401 2500 EK 's-Gravenhage Tel. 070-3792922 (na 14.00 uur) Fax. 070-3793751 5de herziene druk, december 1995 AAN DE LEZER/GEBRUIKER Deze Toelichting is niet bedoeld als een uitputtende behandeling van alle voor- schriften, procedures en dergelijke die gelden voor de handel in dieren en planten waarop de bepalingen van CITES van toepassing zijn, maar dient slechts als leidraad voor belanghebbenden. In twijfelgevallen dient men de relevante wettelijke bepalingen te raadplegen. Ook kan nadere informatie worden verkregen bij het CITES-bureau van de directie Natuurbeheer van het Ministerie van Landbouw, Natuurbeheer en Visserij. Aan deze Toelichting kan dan ook geen enkel recht worden ontleend. Bij de in- en uitvoer van beschermde dieren en planten zijn vaak ook andere do- cumenten vereist dan vermeld in deze Toelichting, zoals bijvoorbeeld veterinai- re documenten, gezondheidscertificaten voor planten en dergelijke. Hiervoor dient men zich tot de daartoe bevoegde instanties te wenden. Deze 5de herziene druk van de Toelichting vervangt de 4de druk van november 1988. Naar beste weten van de samenstellers van deze Toelichting is de tekst hiervan juist. Als de lezer echter onjuistheden of onvolledigheden aantreft, wordt het op prijs gesteld als deze worden gemeld aan het CITES-bureau van de directie Natuurbeheer van het Ministerie van Landbouw, Natuurbeheer en Visserij, Postbus 20401, 2500 EK 's-Gravenhage. -

Organismu Latviskie Nosaukumi (2)
Biosistēmu Terminoloģijas Centra Biļetens 1(1) (2017): 21–51 ISSN 2501-0336 (online) http://www.rpd-science.org/BTCB/V001/BTCB_1_4.pdf © “RPD Science” Citēšanai: BTCB, 2017. Organismu latviskie nosaukumi (2). Biosistēmu Terminoloģijas Centra Biļetens 1(1): 21–51 Organismu latviskie nosaukumi (2) Latvian names of organisms (2) Zinātniskais nosaukums Atbilstība Pēdējā Scientific name Equivalence pārbaude Last verification A Abies gmelinii Rupr. (1845) = Larix gmelinii (Rupr.) Rupr. var. gmelinii 17.12.2016. Abies ledebourii Rupr. (1845) = Larix gmelinii (Rupr.) Rupr. var. gmelinii 17.12.2016. Abies menziesii Mirb. (1825) = Pseudotsuga menziesii (Mirb.) Franco var. menziesii 17.12.2016. Acaciaceae = Fabaceae 27.12.2016. Acalitus brevitarsus (Fockeu, 1890) melnalkšņa maurērce 16.12.2016. Acalitus calycophthirus (Nalepa, 1891) bērzu pumpurērce 16.12.2016. Acalitus essigi (Hassan, 1928) aveņu pangērce 16.12.2016. Acalitus longisetosus (Nalepa, 1892) bērzu sārtā maurērce 16.12.2016. Acalitus phloeocoptes (Nalepa, 1890) plūmju stumbra pangērce 16.12.2016. Acalitus phyllereus (Nalepa, 1919) baltalkšņa maurērce 16.12.2016. Acalitus plicans (Nalepa, 1917) dižskābaržu maurērce 16.12.2016. Acalitus rudis (Canestrini, 1890) bērzu baltā maurērce 16.12.2016. Acalitus stenaspis (Nalepa, 1891) dižskābaržu lapmalērce 16.12.2016. Acalitus vaccinii (Keifer, 1939) melleņu pumpurērce 16.12.2016. Acanthinula aculeata (O. F. Müller, 1774) mazais dzeloņgliemezis 16.12.2016. Acanthinula spinifera Mousson, 1872 Spānijas dzeloņgliemezis 16.12.2016. Acanthocardia echinata (Linnaeus, 1758) dzelkņainā sirsniņgliemene 16.12.2016. Acanthochitona crinita (Pennant, 1777) zaļais bruņgliemis 16.12.2016. Aceria brevipunctatus (Nalepa, 1889) = Aceria campestricola (Frauenfeld, 1865) 16.12.2016. Aceria brevirostris (Nalepa, 1892) ziepenīšu pangērce 16.12.2016. Aceria brevitarsus (Fockeu, 1890) = Acalitus brevitarsus (Fockeu, 1890) 16.12.2016. -

1994 IUCN Red List of Threatened Animals
The lUCN Species Survival Commission 1994 lUCN Red List of Threatened Animals Compiled by the World Conservation Monitoring Centre PADU - MGs COPY DO NOT REMOVE lUCN The World Conservation Union lo-^2^ 1994 lUCN Red List of Threatened Animals lUCN WORLD CONSERVATION Tile World Conservation Union species susvival commission monitoring centre WWF i Suftanate of Oman 1NYZ5 TTieWlLDUFE CONSERVATION SOCIET'' PEOPLE'S TRISr BirdLife 9h: KX ENIUNGMEDSPEaES INTERNATIONAL fdreningen Chicago Zoulog k.J SnuicTy lUCN - The World Conservation Union lUCN - The World Conservation Union brings together States, government agencies and a diverse range of non-governmental organisations in a unique world partnership: some 770 members in all, spread across 123 countries. - As a union, I UCN exists to serve its members to represent their views on the world stage and to provide them with the concepts, strategies and technical support they need to achieve their goals. Through its six Commissions, lUCN draws together over 5000 expert volunteers in project teams and action groups. A central secretariat coordinates the lUCN Programme and leads initiatives on the conservation and sustainable use of the world's biological diversity and the management of habitats and natural resources, as well as providing a range of services. The Union has helped many countries to prepare National Conservation Strategies, and demonstrates the application of its knowledge through the field projects it supervises. Operations are increasingly decentralised and are carried forward by an expanding network of regional and country offices, located principally in developing countries. I UCN - The World Conservation Union seeks above all to work with its members to achieve development that is sustainable and that provides a lasting Improvement in the quality of life for people all over the world. -

Conch 40-6-Text.Indb
Journal of ConChology (2011), Vol.40, no.6 583 TAXONOMIC REVISION, HABITATS AND BIOGEOGRAPHY OF THE LAND SNAIL FAMILY DISCIDAE (GASTROPODA: PULMONATA) IN THE CANARY ISLANDS 1 1 2 3 3 DaViD t. holyoak , geralDine a. holyoak , yurena yanes , Maria r. alonso & Miguel iBáñeZ 1Quinta da Cachopa, Barcoila, 6100–014 Cabeçudo, Portugal. 2Instituto Andaluz de Ciencias de la Tierra (CSIC-Universidad de Granada), Camino del Jueves s/n, 18100, Armilla, Granada, Spain. 3Departamento de Biologia Animal, Universidad de La Laguna, E-38206 La Laguna, Tenerife, Islas Canarias, Spain. Abstract The endemic Macaronesian Canaridiscus, provisionally placed in the Discidae genus Atlantica, are closely linked to the peculiar laurel forest habitat of these islands. Knowledge of Atlantica (Canaridiscus) is increased here with the descrip- tion, for the first time, of the genital system of three more of its species. The epiphallus is apparently lacking and the penis is much longer than that known from any of the Discidae of Europe and North America; generally it is too large to be more than partly accommodated in the distal female genital tract. Keraea has been regarded as an endemic Macaronesian genus of Discidae known only from a few shells, but its Madeiran species has been identified as a Trochulus (Hygromiidae) and its type species (from Tenerife) also matches Hygromiidae, not Discidae. The origin and relationships of the Macaronesian Discidae are discussed. The simplest explanatory model is apparently that they represent the last living relicts of a more diverse fauna of Discidae that lived in Europe during the Tertiary; Atlantica (Canaridiscus) was probably isolated early on from the remaining Discidae. -

European Red List of Non-Marine Molluscs Annabelle Cuttelod, Mary Seddon and Eike Neubert
European Red List of Non-marine Molluscs Annabelle Cuttelod, Mary Seddon and Eike Neubert European Red List of Non-marine Molluscs Annabelle Cuttelod, Mary Seddon and Eike Neubert IUCN Global Species Programme IUCN Regional Office for Europe IUCN Species Survival Commission Published by the European Commission. This publication has been prepared by IUCN (International Union for Conservation of Nature) and the Natural History of Bern, Switzerland. The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN, the Natural History Museum of Bern or the European Union concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN, the Natural History Museum of Bern or the European Commission. Citation: Cuttelod, A., Seddon, M. and Neubert, E. 2011. European Red List of Non-marine Molluscs. Luxembourg: Publications Office of the European Union. Design & Layout by: Tasamim Design - www.tasamim.net Printed by: The Colchester Print Group, United Kingdom Picture credits on cover page: The rare “Hélice catalorzu” Tacheocampylaea acropachia acropachia is endemic to the southern half of Corsica and is considered as Endangered. Its populations are very scattered and poor in individuals. This picture was taken in the Forêt de Muracciole in Central Corsica, an occurrence which was known since the end of the 19th century, but was completely destroyed by a heavy man-made forest fire in 2000.