Idaho Goldenweed (Tonestus Aberrans) Predicted Suitable Habitat Modeling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

The Jepson Manual: Vascular Plants of California, Second Edition Supplement II December 2014
The Jepson Manual: Vascular Plants of California, Second Edition Supplement II December 2014 In the pages that follow are treatments that have been revised since the publication of the Jepson eFlora, Revision 1 (July 2013). The information in these revisions is intended to supersede that in the second edition of The Jepson Manual (2012). The revised treatments, as well as errata and other small changes not noted here, are included in the Jepson eFlora (http://ucjeps.berkeley.edu/IJM.html). For a list of errata and small changes in treatments that are not included here, please see: http://ucjeps.berkeley.edu/JM12_errata.html Citation for the entire Jepson eFlora: Jepson Flora Project (eds.) [year] Jepson eFlora, http://ucjeps.berkeley.edu/IJM.html [accessed on month, day, year] Citation for an individual treatment in this supplement: [Author of taxon treatment] 2014. [Taxon name], Revision 2, in Jepson Flora Project (eds.) Jepson eFlora, [URL for treatment]. Accessed on [month, day, year]. Copyright © 2014 Regents of the University of California Supplement II, Page 1 Summary of changes made in Revision 2 of the Jepson eFlora, December 2014 PTERIDACEAE *Pteridaceae key to genera: All of the CA members of Cheilanthes transferred to Myriopteris *Cheilanthes: Cheilanthes clevelandii D. C. Eaton changed to Myriopteris clevelandii (D. C. Eaton) Grusz & Windham, as native Cheilanthes cooperae D. C. Eaton changed to Myriopteris cooperae (D. C. Eaton) Grusz & Windham, as native Cheilanthes covillei Maxon changed to Myriopteris covillei (Maxon) Á. Löve & D. Löve, as native Cheilanthes feei T. Moore changed to Myriopteris gracilis Fée, as native Cheilanthes gracillima D. -
1 C M 2 B H 3 B M 4 C M 5 B L 6 B M 7 B M 8
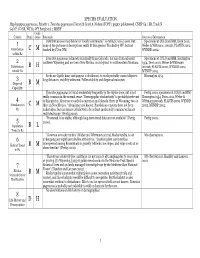
SPECIES EVALUATION Haplopappus pygmaeus, Priority 1. Tonestus pygmaeus (Torrey & Gray) A. Nelson (TOPY). pygmy goldenweed. CNHP G4 / SR, Track N G4 N?. CO SR, WY S1. WY Peripheral 2 MBNF Confi- Criteria Rank dence Rationale Sources of Information Distribution (see map below) is “nearly continuous,” so rating C was chosen, but Specimens at COLO and RM, Dorn 2001, 1 none of the pictures or descriptions really fit this species. Tracked by WY, but not Weber & Wittmann 2001ab, PLANTS 2002, Distribution C M tracked by CO or NM. WYNDD 2002. within R2 Tonestus pygmaeus is known principally from Colorado, but also from adjacent Specimens at COLO and RM, Harrington 2 southern Wyoming and northern New Mexico, and disjunct in southwestern Montana. 1954, Dorn 2001, Weber & Wittmann Distribution B H 2001ab, PLANTS 2002, WYNDD 2002, outside R2 MTNHP 2002. Seeds are lightly hairy and pappus is deciduous, so seeds probably cannot disperse Harrington 1954. 3 long distances; viability unknown. Pollen viability and dispersal unknown. Dispersal B M Capability Tonestus pygmaeus is found moderately frequently in the Alpine zone, but is not Fertig 2000, specimens at COLO and RM, really common in the normal sense. “Demographic stochasticity” is probably irrelevant Harrington 1954, Dorn 2001, Weber & 4 to this species. About 50 recorded occurrences in Colorado, three in Wyoming, two or Wittmann 2001ab, PLANTS 2002, WYNDD Abundance in C M three in New Mexico. “Abundance not known. Population censuses have not been 2002, MTNHP 2002. R2 undertaken, but the species is believed to be at least moderately common within its restricted range” (Fertig 2000). -

Reclassification of North American Haplopappus (Compositae: Astereae) Completed: Rayjacksonia Gen
AmericanJournal of Botany 83(3): 356-370. 1996. RECLASSIFICATION OF NORTH AMERICAN HAPLOPAPPUS (COMPOSITAE: ASTEREAE) COMPLETED: RAYJACKSONIA GEN. NOV.1 MEREDITH A. LANE2 AND RONALD L. HARTMAN R. L. McGregor Herbarium(University of Kansas NaturalHistory Museum Division of Botany) and Departmentof Botany,University of Kansas, Lawrence, Kansas 66047-3729; and Rocky MountainHerbarium, Department of Botany,University of Wyoming,Laramie, Wyoming82071-3165 Rayjacksonia R. L. Hartman& M. A. Lane, gen. nov. (Compositae: Astereae), is named to accommodate the "phyllo- cephalus complex," formerlyof Haplopappus Cass. sect. Blepharodon DC. The new combinationsare R. phyllocephalus (DC.) R. L. Hartman& M. A. Lane, R. annua (Rydb.) R. L. Hartman& M. A. Lane, and R. aurea (A. Gray) R. L. Hartman & M. A. Lane. This transfercompletes the reclassificationof the North American species of Haplopappus sensu Hall, leaving that genus exclusively South American.Rayjacksonia has a base chromosomenumber of x = 6. Furthermore,it shares abruptlyampliate disk corollas, deltatedisk style-branchappendages, and corolla epidermalcell type,among other features,with Grindelia, Isocoma, Olivaea, Prionopsis, Stephanodoria, and Xanthocephalum.Phylogenetic analyses of morphologicaland chloroplastDNA restrictionsite data, taken together,demonstrate that these genera are closely related but distinct. Key words: Astereae; Asteraceae; Compositae; Haplopappus; Rayjacksonia. During the past seven decades, taxonomic application lopappus sensu Hall (1928) are reclassifiedand are cur- -

U.S. Department of the Interior Bureau of Land Management Battle Battle Mountain Office District
BLM U.S. Department of the Interior Bureau of Land Management Battle Mountain District Office Mountain Battle Gold Bar Exploration Project Resource Report 23 Appendices DOI-BLM-NV-B010-2018-0038-EA Preparing Office Battle Mountain District Office 50 Bastian Road Battle Mountain, NV 89820 December 2018 GOLD BAR RESOURCE REPORT 23 APPENDICES Appendix A – Summary of Findings Appendix B – BLM Sensitive Species List APPENDIX A SUMMARY OF FINDINGS Summary of Environmental Consequences RR # Resource Subtopic Context Duration Intensity Significance Summary Exploration activities will be exploratory and not extractive. Surface disturbances during exploration activities will consist primarily of the creation of new exploration roads, drill pits, and sumps. At any given time within the 17,316-acre EPO Area, a maximum of 100 acres of unreleased Proposed Action-related disturbance will exist. Release of reclaimed lands 3 Geology & Minerals --- Localized Short-term Negligible Not Significant could occur as early as the third growing season as stated in the BLM and NDEP bond release policy. Overall, it is anticipated that the program will be conducted over 10 years with total unreleased disturbance not exceeding 100 acres at any point in time and with a total project reclaimed disturbance not exceeding 200 acres (based on concurrent reclamation being released for future phases of exploration activities). Changes in runoff distribution would be negligible and the overall effect on surface water resource would be negligible. The Proposed Action is expected to have a negligible effect on snowmelt recharge to shallow groundwater within EPO Surface Water Localized Short-term Negligible Not Significant Area. BMPs to control stormwater, sediment erosion, and protect surface water resources from pollution related to surface disturbance activities would be implemented to reduce the potential for temporary effects to water quality. -

Volume 4 Number 1 Oregon State University February I998 , ,.;
Volume 4 Number 1 Oregon State University February I998 Clay Gautier Checklist: Asteraceac by Rhoda Love by Scon Sundherg und Kenton L. Chambers We are pleased to announce the completion of the Clay Gaulier is fhe newatmember of the &gon checklist of Oregon composites (Astmceae)by the AtlasEoject Leaders group. Hehas volunteered with Oregon Vascular mant CbecklktProject. The Scott Sundberg and o& since 1996. 44Clayhas been Asteraceae checklist was written by Eenton.chambers instrumental in deveIoping an ektmnic version of the and Scott Sundberg andis currently being reviewed by OregonPlant Aflas that willbe fully intmmfive and Checklist project members. It is a pomon of thc Oregon accessibIeto the public via tb Iaremeff' says Scott. va.scular 61At Checklist, which iQpreparatoryto a new Clay, who commuIes'ta~0SUhmF&gene,brings a Flora of Oregon. The Asteraceae Checklist includes special combination of skills tbfhis task: underpdLta@ accepted names of all Oregon tam [species, subspecies Ad graduate workcinec~logyand 12 years of experience and varieties) growing outside of cultivation, as well as as a proEssional software'developer. common m,plant origin (native or introduced) and Clay was born in San Francis~oin the niid-B%es comments on taxonomic problems and hybridization. and grew np in California and Nevada. He says, "My Each recod iq backed up by annotated herbarium wife, Gail Baker, and I both went tb Redwood High specimens or by reference to a published monograph or School in Marin Countv when I was a freshman, but we revision. The, ,.; accepted. namcs.will.be mssslerenced to didn't mypcq~y~~+l ~:~?,l$er in B&.o@gPli; . -

Annotated Checklist of Vascular Flora, Cedar Breaks National
National Park Service U.S. Department of the Interior Natural Resource Program Center Annotated Checklist of Vascular Flora Cedar Breaks National Monument Natural Resource Technical Report NPS/NCPN/NRTR—2009/173 ON THE COVER Peterson’s campion (Silene petersonii), Cedar Breaks National Monument, Utah. Photograph by Walter Fertig. Annotated Checklist of Vascular Flora Cedar Breaks National Monument Natural Resource Technical Report NPS/NCPN/NRTR—2009/173 Author Walter Fertig Moenave Botanical Consulting 1117 W. Grand Canyon Dr. Kanab, UT 84741 Editing and Design Alice Wondrak Biel Northern Colorado Plateau Network P.O. Box 848 Moab, UT 84532 February 2009 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientifi c community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peer-reviewed to ensure that the information is scientifi cally credible, technically accurate, appropriately written for the intended audience, and is designed and published in a professional manner. The Natural Resource Technical Report series is used to disseminate the peer-reviewed results of scientifi c studies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service’s mission. The reports provide contributors with a forum for displaying comprehensive data that are often deleted from journals because of page limitations. Current examples of such reports include the results of research that addresses natural resource management issues; natural resource inventory and monitoring activities; resource assessment reports; scientifi c literature reviews; and peer- reviewed proceedings of technical workshops, conferences, or symposia. -

Asters of Yesteryear (Updated April 2018)
Asters of Yesteryear (Updated April 2018) About this Update: The document was originally posted in a shorter version, to accompany the brief article "Where Have all our Asters Gone?" in the Fall 2017 issue of Sego Lily. In that version it consisted simply of photos of a number of plants that had at some time been included in Aster but that no longer are, as per Flora of North America. In this version I have added names to the photos to indicate how they have changed since their original publication: Date and original name as published (Basionym) IF name used in Intermountain Flora (1994) UF name used in A Utah Flora (1983-2016) FNA name used in Flora of North America (2006) I have also added tables to show the renaming of two groups of species in the Astereae tribe as organized in Intermountain Flora. Color coding shows how splitting of the major genera largely follows fault lines already in place No color Renamed Bright Green Conserved Various Natural groupings $ Plant not in Utah It is noteworthy how few species retain the names used in 1994, but also how the renaming often follows patterns already observed. Asters of Yesteryear (Updated April 2018) Here are larger photos (16 inches wide or tall at normal screen resolution of 72 dpi) of the plants shown in Sego Lily of Fall 2017, arranged by date of original publication. None of them (except Aster amellus on this page) are now regarded as true asters – but they all were at one stage in their history. Now all are in different genera, most of them using names that were published over 100 years ago. -

Cliff Paintbrush (Castilleja Rupicola) in Canada
PROPOSED Species at Risk Act Recovery Strategy Series Adopted under Section 44 of SARA Recovery Strategy for the Cliff Paintbrush (Castilleja rupicola) in Canada Cliff Paintbrush 2016 Recommended citation: Environment Canada. 2016. Recovery Strategy for the Cliff Paintbrush (Castilleja rupicola) in Canada [Proposed]. Species at Risk Act Recovery Strategy Series. Environment Canada, Ottawa. 20 pp. + Annex. For copies of the recovery strategy, or for additional information on species at risk, including the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk (SAR) Public Registry1. Cover illustration: © Ross Vennesland Également disponible en français sous le titre « Programme de rétablissement de la castilléjie des rochers (Castilleja rupicola) au Canada [Proposition] » © Her Majesty the Queen in Right of Canada, represented by the Minister of the Environment, 2016. All rights reserved. ISBN Catalogue no. Content (excluding the illustrations) may be used without permission, with appropriate credit to the source. 1 http://sararegistry.gc.ca/default.asp?lang=En&n=24F7211B-1 RECOVERY STRATEGY FOR THE CLIFF PAINTBRUSH (Castilleja rupicola) IN CANADA 2016 Under the Accord for the Protection of Species at Risk (1996), the federal, provincial, and territorial governments agreed to work together on legislation, programs, and policies to protect wildlife species at risk throughout Canada. In the spirit of cooperation of the Accord, the Government of British Columbia has given permission to the Government of Canada to adopt the Recovery Strategy for Cliff Paintbrush (Castilleja rupicola) in British Columbia (Part 2) under Section 44 of the Species at Risk Act (SARA). -

Sensitive Species That Are Not Listed Or Proposed Under the ESA Sorted By: Major Group, Subgroup, NS Sci
Forest Service Sensitive Species that are not listed or proposed under the ESA Sorted by: Major Group, Subgroup, NS Sci. Name; Legend: Page 94 REGION 10 REGION 1 REGION 2 REGION 3 REGION 4 REGION 5 REGION 6 REGION 8 REGION 9 ALTERNATE NATURESERVE PRIMARY MAJOR SUB- U.S. N U.S. 2005 NATURESERVE SCIENTIFIC NAME SCIENTIFIC NAME(S) COMMON NAME GROUP GROUP G RANK RANK ESA C 9 Anahita punctulata Southeastern Wandering Spider Invertebrate Arachnid G4 NNR 9 Apochthonius indianensis A Pseudoscorpion Invertebrate Arachnid G1G2 N1N2 9 Apochthonius paucispinosus Dry Fork Valley Cave Invertebrate Arachnid G1 N1 Pseudoscorpion 9 Erebomaster flavescens A Cave Obligate Harvestman Invertebrate Arachnid G3G4 N3N4 9 Hesperochernes mirabilis Cave Psuedoscorpion Invertebrate Arachnid G5 N5 8 Hypochilus coylei A Cave Spider Invertebrate Arachnid G3? NNR 8 Hypochilus sheari A Lampshade Spider Invertebrate Arachnid G2G3 NNR 9 Kleptochthonius griseomanus An Indiana Cave Pseudoscorpion Invertebrate Arachnid G1 N1 8 Kleptochthonius orpheus Orpheus Cave Pseudoscorpion Invertebrate Arachnid G1 N1 9 Kleptochthonius packardi A Cave Obligate Pseudoscorpion Invertebrate Arachnid G2G3 N2N3 9 Nesticus carteri A Cave Spider Invertebrate Arachnid GNR NNR 8 Nesticus cooperi Lost Nantahala Cave Spider Invertebrate Arachnid G1 N1 8 Nesticus crosbyi A Cave Spider Invertebrate Arachnid G1? NNR 8 Nesticus mimus A Cave Spider Invertebrate Arachnid G2 NNR 8 Nesticus sheari A Cave Spider Invertebrate Arachnid G2? NNR 8 Nesticus silvanus A Cave Spider Invertebrate Arachnid G2? NNR -

Asteraceae)§ Karin M.Valant-Vetscheraa and Eckhard Wollenweberb,*
Chemodiversity of Exudate Flavonoids in Seven Tribes of Cichorioideae and Asteroideae (Asteraceae)§ Karin M.Valant-Vetscheraa and Eckhard Wollenweberb,* a Department of Plant Systematics and Evolution Ð Comparative and Ecological Phytochemistry, University of Vienna, Rennweg 14, A-1030 Wien, Austria b Institut für Botanik der TU Darmstadt, Schnittspahnstrasse 3, D-64287 Darmstadt, Germany. E-mail: [email protected] * Author for correspondence and reprint requests Z. Naturforsch. 62c, 155Ð163 (2007); received October 26/November 24, 2006 Members of several genera of Asteraceae, belonging to the tribes Mutisieae, Cardueae, Lactuceae (all subfamily Cichorioideae), and of Astereae, Senecioneae, Helenieae and Helian- theae (all subfamily Asteroideae) have been analyzed for chemodiversity of their exudate flavonoid profiles. The majority of structures found were flavones and flavonols, sometimes with 6- and/or 8-substitution, and with a varying degree of oxidation and methylation. Flava- nones were observed in exudates of some genera, and, in some cases, also flavonol- and flavone glycosides were detected. This was mostly the case when exudates were poor both in yield and chemical complexity. Structurally diverse profiles are found particularly within Astereae and Heliantheae. The tribes in the subfamily Cichorioideae exhibited less complex flavonoid profiles. Current results are compared to literature data, and botanical information is included on the studied taxa. Key words: Asteraceae, Exudates, Flavonoids Introduction comparison of accumulation trends in terms of The family of Asteraceae is distributed world- substitution patterns is more indicative for che- wide and comprises 17 tribes, of which Mutisieae, modiversity than single compounds. Cardueae, Lactuceae, Vernonieae, Liabeae, and Earlier, we have shown that some accumulation Arctoteae are grouped within subfamily Cichori- tendencies apparently exist in single tribes (Wol- oideae, whereas Inuleae, Plucheae, Gnaphalieae, lenweber and Valant-Vetschera, 1996). -

2008-2009 NARGS Seed List
Abelmoschus-Allium 1 Abelmoschus manihot white-yellow to 2m 204 72 Agastache rugosa 'Golden Jubilee' blue-purple/ 2 Abutilon palmeri orange 1.5-2m 16 chartreuse lvs 100cm 92 3 sp orange 35cm 247 73 rupestris orange 80cm 94 4 Acacia pravissima yellow to 8m 34 74 rupestris pink-orange 60cm 215 5 Acaena myriophylla green to 30cm 52 75 scrophulariifolia purple 60-150cm 226 6 saccaticupula blue-gray lvs 5-10cm 278 76 Agoseris retrorsa yellow 15-65cm 241 7 sericea purple fls/silver lvs 6-25cm 70 77 Albuca canadensis white 1.5-1.8m 267 8 Acantholimon armenum pink-white 10-20cm 50 78 shawii yellow 45cm > 9 hohenackeri pink 5-10cm 274 79 shawii yellow 25cm 87 10 kotschyi ssp laxispicatum pink 5-10cm 274 80 sp ex JCA 15856 white/green 10cm 113 11 Acanthus sp white 100cm 272 81 Alcea excubita ex Turkey white 40-100cm 105 12 Acer japonicum to 10m 247 82 rosea 'Nigra' dark maroon 1.5-2m 88 13 palmatum v dissectum to 3m 247 83 Aletris farinosa white 40-100cm 10 14 pseudoplatanus to 40m 247 84 Alkanna graeca yellow 30-45cm 103 15 Achillea ageratifolia ssp aizoon white 15cm 30 85 Allium abramsii rose-purple 6-15cm 11 16 ambrosiaca white 10-15cm 70 176 86 aflatunense purple 1m 109 17 sintenisii white 10-25cm 70 87 bolanderi reddish purple 10-35cm 11 198 18 teretifolia white to 35cm 65 88 caeruleum blue 20-30cm 174 282 19 tomentosa yellow 20-30cm 66 89 caesium violet-blue 35-50cm 17 231 20 Acis autumnalis white w/pink base 8-15cm > 90 campanulatum rose-purple 10-30cm 11 21 nicaeensis white/green 5-18cm > 91 carinatum ssp pulchellum lavender 30-50cm 168