Characterization and Utilization of Cement Kiln Dusts (Ckds) As Partial Replacements of Portland Cement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Cement Data Sheet
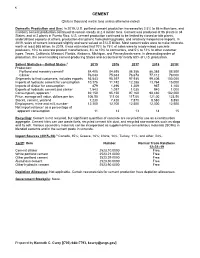
42 CEMENT (Data in thousand metric tons unless otherwise noted) Domestic Production and Use: In 2019, U.S. portland cement production increased by 2.5% to 86 million tons, and masonry cement production continued to remain steady at 2.4 million tons. Cement was produced at 96 plants in 34 States, and at 2 plants in Puerto Rico. U.S. cement production continued to be limited by closed or idle plants, underutilized capacity at others, production disruptions from plant upgrades, and relatively inexpensive imports. In 2019, sales of cement increased slightly and were valued at $12.5 billion. Most cement sales were to make concrete, worth at least $65 billion. In 2019, it was estimated that 70% to 75% of sales were to ready-mixed concrete producers, 10% to concrete product manufactures, 8% to 10% to contractors, and 5% to 12% to other customer types. Texas, California, Missouri, Florida, Alabama, Michigan, and Pennsylvania were, in descending order of production, the seven leading cement-producing States and accounted for nearly 60% of U.S. production. Salient Statistics—United States:1 2015 2016 2017 2018 2019e Production: Portland and masonry cement2 84,405 84,695 86,356 86,368 88,500 Clinker 76,043 75,633 76,678 77,112 78,000 Shipments to final customers, includes exports 93,543 95,397 97,935 99,406 100,000 Imports of hydraulic cement for consumption 10,376 11,742 12,288 13,764 15,000 Imports of clinker for consumption 879 1,496 1,209 967 1,100 Exports of hydraulic cement and clinker 1,543 1,097 1,035 940 1,000 Consumption, apparent3 92,150 95,150 97,160 98,480 102,000 Price, average mill value, dollars per ton 106.50 111.00 117.00 121.00 123.50 Stocks, cement, yearend 7,230 7,420 7,870 8,580 8,850 Employment, mine and mill, numbere 12,300 12,700 12,500 12,300 12,500 Net import reliance4 as a percentage of apparent consumption 11 13 13 14 15 Recycling: Cement is not recycled, but significant quantities of concrete are recycled for use as a construction aggregate. -

Pre-Industrial Lime Kilns
Pre-industrial Lime Kilns On 1st April 2015 the Historic Buildings and Monuments Commission for England changed its common name from English Heritage to Historic England. We are now re-branding all our documents. Although this document refers to English Heritage, it is still the Commission's current advice and guidance and will in due course be re-branded as Historic England. Please see our website for up to date contact information, and further advice. We welcome feedback to help improve this document, which will be periodically revised. Please email comments to [email protected] We are the government's expert advisory service for England's historic environment. We give constructive advice to local authorities, owners and the public. We champion historic places helping people to understand, value and care for them, now and for the future. HistoricEngland.org.uk/advice Introductions to Heritage Assets Pre-industrial Lime Kilns May 2011 Fig. 1. Ground plans showing the main types of Romano-British lime kilns known in England. A – Periodic or flare kiln. B – Irregular kiln. C – Pit or ‘clamp’ kiln. INTRODUCTION A lime kiln was a structure used to manufacture lime (Figure 1). During the Anglo-Saxon period buildings (calcium oxide) by burning calcium carbonate at were usually of timber construction and, although lime temperatures above 900°C. The calcium carbonate may have been used for white-washing high status burned (or ‘calcined’) was commonly limestone or buildings, the demand for it was very low. This changed chalk, but occassionally other materials such as oyster in the medieval period (1066-1485), when large or egg shells were used. -

53652 02 MG Lime Kiln Leaflet 8Pp:Layout 2
Slaked lime. What happened to the industry? The lime kilns today Discover What was To make clay soils more By the 1880s the lime trade was Today the lime kilns stand as a monument to the industrial era in a workable and to neutralise in decline and by 1900 seems to place not usually associated with such activity. In recent times, work the burnt lime acid soil.** have ceased production. Activity has been carried out by the National Trust which has involved parts of To make whitewash, had only been sporadic through the kilns being reinforced and altered. This is most evident around the used for? mortar and plaster.** the final years of the nineteenth The Castle Point south western pot, where the brick walls have been removed from century. On the 17th September above the draw arches and concrete lintels have been installed. Burnt lime from Lindisfarne was To destroy odours in mass probably used primarily in burials.** 1883, the Agnes left the Staithes; In 2010, the first phase of important improvements to access and agriculture. The alkali-rich slaked the last ship to depart Holy interpretation began. The old fences were improved to prevent sheep To make bleaching powder, lime kilns lime was perfect for neutralising Island laden with lime, This ship, a disinfectant.* from gaining access to the kilns, and a floor was laid in the central acidic soil and so improving along with others of Nicoll’s passageway. A new public access gate was also installed. fertility. It is also likely that some fleet, did return in the next few To make caustic soda used to Funding for this project came from National Trust Property Raffle sales of the slaked lime was used in make soap.* years but only, it seems, to in the Castle, Gift Aid on Entry money from visitors. -

Cement & Lime Manufacture 1968 Vol.41 No.2
C EM E NT & LI M E MANUFACTURE P rice Is. 6d. per Copy. Annual subscriptio ns: Inland 9s. post free. VOL. XLI. No. 2 MARCH, J968 Overseas lis. 6d . incl udi ng postage. Canada and U.S.A. 1.80 dollars incl. post. U.K. R ~ ;>re,ent.llyes:- CO NTI N ENTAL ENG I NEE RING CO. LTD., London Road, Ascot, Berks. Tel. No : Winkfield Row 20 II PAGE ii CE M ENT AND LIM E MANU FACTU RE M ARCI-II % R FULLER-DRACCO GLASS CLOTH FILTERS FOR THE CEMENT INDUSTRY * For completely dust-free cement production both kilns and clinker coolers should have Fuller- Dracco Glass-Bag filters. * Fuller-Dracco Glass-Bag filters assure virtually 100% dust collection from hot gases. * Glass bags withstand continuous temperatures of up to 550°F. * Reverse air and sonic cleaning prolong bag life. * Bag-houses can be designed for vacuum or pressure operation and for any capacity. * The largest suction type Glass Cloth Collector in the world is a Dracco installation handling 350,000 c.f.m. dry process kiln gas. Glass cloth gas cleaning has proved the most effec tive means of trapping hot dust i n cement plants and Fuller's extensive experience in designing and CONSTANTIN developing Glass-Bag Dust Collectors for many (ENGINEERS) LTD_ applications makes us best qualified to build your next RADNOR HOUSE . LONDON ROAD' NORBURY collecting system. LONDON ' S.W.16 <D 01-784 1571 ; Fuller-Dracco Glass-Bag dust collectors operate at intake temperatures of up to 550°F without pre ECDNSTANTIN CONSTANTIN cooling and we can offer a variety of bag cleaning SA ESPANOLA SJI techniques to suit your operating requ irements. -

Use of Cement Kiln Dust for Subgrade Stabilization
Report No. KS-04-3 FINAL REPORT USE OF CEMENT KILN DUST FOR SUBGRADE STABILIZATION Robert L. Parsons, Ph.D., P.E. Elizabeth Kneebone University of Kansas Justin P. Milburn Continental Consulting Engineers, Inc. OCTOBER 2004 KANSAS DEPARTMENT OF TRANSPORTATION Division of Operations Bureau of Materials and Research 1 Report No. 2 Government Accession No. 3 Recipient Catalog No. KS-04-3 4 Title and Subtitle 5 Report Date USE OF CEMENT KILN DUST FOR SUBGRADE STABILIZATION October 2004 6 Performing Organization Code 7 Author(s) 8 Performing Organization Report Robert L. Parsons, Ph.D., P.E., Elizabeth Keebone, both of University of No. Kansas, and Justin P. Milburn, Continental Consulting Engineers, Inc. 9 Performing Organization Name and Address 10 Work Unit No. (TRAIS) University of Kansas 1530 West 15th Street; Learned Hall 11 Contract or Grant No. Lawrence, Kansas 66045-7609 C1378 12 Sponsoring Agency Name and Address 13 Type of Report and Period Kansas Department of Transportation Covered Bureau of Materials and Research Final Report 700 SW Harrison Street October 2002 to July 2004 Topeka, Kansas 66603-3754 14 Sponsoring Agency Code RE-0331-01 15 Supplementary Notes Fly Ash Management. L.L.C., of Topeka, Kansas also provided funding for this project. For more information on this report, please write to address in block 9. 16 Abstract Poor subgrade soil conditions can result in inadequate pavement support and reduce pavement life. Soils may be improved through the addition of chemical or cementitious additives. These chemical additives range from waste products to manufactured materials and include lime, Class C fly ash, Portland cement, cement kiln dust from pre-calciner and long kiln processes, and proprietary chemical stabilizers. -

Lime Kilns in Worcestershire
Lime Kilns in Worcestershire Nils Wilkes Acknowledgements I first began this project in September 2012 having noticed a number of limekilns annotated on the Ordnance Survey County Series First Edition maps whilst carrying out another project for the Historic Environment Record department (HER). That there had been limekilns right across Worcestershire was not something I was aware of, particularly as the county is not regarded to be a limestone region. When I came to look for books or documents relating specifically to limeburning in Worcestershire, there were none, and this intrigued me. So, in short, this document is the result of my endeavours to gather together both documentary and physical evidence of a long forgotten industry in Worcestershire. In the course of this research I have received the help of many kind people. Firstly I wish to thank staff at the Historic Environmental Record department of the Archive and Archaeological Service for their patience and assistance in helping me develop the Limekiln Database, in particular Emma Hancox, Maggi Noke and Olly Russell. I am extremely grateful to Francesca Llewellyn for her information on Stourport and Astley; Simon Wilkinson for notes on Upton-upon-Severn; Gordon Sawyer for his enthusiasm in locating sites in Strensham; David Viner (Canal and Rivers Trust) in accessing records at Ellesmere Port; Bill Lambert (Worcester and Birmingham Canal Trust) for involving me with the Tardebigge Limekilns Project; Pat Hughes for her knowledge of the lime trade in Worcester and Valerie Goodbury -

Carbon Dioxide Control Technologies for the Cement Industry Volker Hoenig, Düsseldorf, Germany
Carbon Dioxide Control Technologies for the Cement Industry Volker Hoenig, Düsseldorf, Germany GCEP Workshop “Carbon Management in Manufacturing Industries” Stanford University, 15/16 April 2008 Carbon Dioxide Control Technologies for the Cement Industry 1. Introduction 2. The cement clinker burning process 3. Assessment of carbon dioxide control technologies 3.1 Pre-combustion technologies 3.2 Oxyfuel technology 3.3 Post-combustion technologies 4. Preliminary research results (Oxyfuel technology) 4.1 Impact on raw meal decarbonation 4.2 Modeling of the clinker burning process with Oxyfuel operation The German Cement Works Association (VDZ) Activities: • Mortar and concrete • Chemistry and mineralogy • Environmental expertise • Plant technology investigations • Environmental measurements • Certification • Knowledge transfer ↑ The Research Institute of the Cement Industry, Düsseldorf / Germany For further information: www.vdz-online.de The European Cement Research Academy (ECRA) • has been founded by VDZ in 2004 • Members (> 40) are cement companies and associations from Europe, Asia, Australia, US • objectives Æ know how transfer Æ joint research • activities Æ seminars and workshops Æ research programmes - carbon capture technologies for the cement industry - continuous measurement of biomass CO2 in stack gases For further information: www.ecra-online.org Carbon Dioxide Control Technologies for the Cement Industry 1. Introduction 2. The cement clinker burning process 3. Assessment of carbon dioxide control technologies 3.1 Pre-combustion -

Fossil Beach Cement Works Heritage Study
Special Council Meeting - Item 2.2 Attachment 2 Monday, 1 August, 2011 BIOSIS R E S E A R C H Fossil Beach Cement Works Heritage Study April 2011 ‘A view of Fossil Beach in 1872’, oil painting by R Scott, in the collection of the Mornington Peninsula Regional Gallery. Natural & BIOSISCultural Heritage R E S E Consultants A R C H 38 Bertie Street (PO1 Box 489) Port Melbourne Victoria 3207 Special Council Meeting - Item 2.2 Attachment 2 Monday, 1 August, 2011 BIOSIS R E S E A R C H Fossil Beach Cement Works Heritage Study April 2011 Gary Vines & Geoff Yugovic Report for Mornington Peninsula Shire Ballarat: 449 Doveton Street North Ballarat 3350 Ph: (03) 5331 7000 Fax: (03) 5331 7033 email: [email protected] Melbourne: 38 Bertie Street Port Melbourne 3207 Ph: (03) 9646 9499 Fax: (03) 9646 9242 email: [email protected] Biosis Research Project – 11255 Date of Completion – 12/05/2011 Canberra: Unit 16/2 Yallourn Street Fyshwick 2609 ISBN 978-1-921405-10-5 Ph: (02) 6228 1599 Fax: (02) 6280 8752 email: [email protected] Sydney: 18-20 Mandible Street Alexandria 2015 Ph: (02) 9690 2777 Fax: (02) 9690 2577 email: [email protected] Wangaratta: PO Box 943 Wangaratta 3676 Ph: (03) 5721 9453 Fax: (03) 5721 9454 email: [email protected] Wollongong: 8 Tate Street Wollongong 2500 Ph: (02) 4229 5222 Fax: (02) 4229 55 email: [email protected] Biosis Research Pty. Ltd. This document is and shall remain the property of Biosis Research Pty. -

Lime Burning in Westmorland
Victoria County History of Cumbria Project: Interim Draft [Note: This is an interim draft and should not be cited without first consulting the VCH Cumbria project: for contact details, see http://www.cumbriacountyhistory.org.uk/] Author: David Johnson LIME BURNING IN WESTMORLAND INTRODUCTION Lime has been produced in Britain since at least Roman times, though there appear to be some hints of its use prior to that.1 The burning of lime in the medieval era has been attested by archaeological excavation of a sow, or clamp, kiln on the southern flanks of Ingleborough, radiocarbon dated to the twelfth or thirteenth century, and of a similar sow kiln in the Forest of Bowland radiocarbon dated to the thirteenth century. In both cases, given that the sites in question were in very remote agricultural locations, the lime was probably destined for use on the land.2 No such evidence has been recorded for Westmorland but it goes without saying that every stone-built structure – church, monastery, bridge, manorial hall or baronial castle – would have needed lime in medieval times and it was common practice to construct a simple kiln at the place where lime mortar was needed. Indeed, this would also have applied in the pre-Conquest era. Kilns prior to the very late seventeenth century were not the masonry-fronted kilns surviving 1 Strabo (d. AD 23) wrote of tribesmen in what is now north-east Wales coating their hair with limewash and drawing it back thickened with lime so that it differed ‘in no way from a horse’s mane’ (I. -

SAFETY DATA SHEET Lime Kiln Dust
MISSISSIPPI LIME COMPANY - SAFETY DATA SHEET Section 1: Identification Lime Kiln Dust Product Line Lime Kiln Dust, LKD, Code L, "Codel" Product Uses Soil stabilization, conditioner, additive, filler, pH adjustment, DOT specifications. Manufacturer Mississippi Lime Company 16147 US Highway 61, Ste Genevieve, MO 63670 24 Hour Emergency Contact Number: (800) 437-5463 Section 2: Hazard(s) Identification Signal Word DANGER ! NFPA HMIS HEALTH - 3 0 FLAMMABILITY - 0 3 1 PHYSICAL HAZ - 1 E PER. PROTECTION- E HARMFUL IF SWALLOWED OR INHALED. CAUSES BURNS TO SKIN AND EYES. CAUSES SEVERE DESCRIPTION IRRITATION TO RESPIRATORY TRACT. H 315: Causes skin irritation. H 318: Causes serious eye damage. Hazard H 333: May be harmful if inhaled.. H 335: May cause respiratory irritation H 351: Suspected of causing cancer PREVENTION P 102: Keep out of reach of children. P 261: Avoid breathing dust. P 280: Wear protective gloves/protective clothing/eye protection/face protection. P 402: Store in dry place. RESPONSE P 302 + P 352: IF ON SKIN: Wash with plenty of soap and water. P 304 + P 340: IF INHALED: Remove victim to fresh air and keep at rest and comfortable. Statements P 305 + P 351: IF IN EYES: Rinse cautiously with water for several minutes. Precautionary Precautionary P 305 + P 337 + P 313: IF IN EYES: If eye irritation persists, Get medical advice/attention P 301 + P 330 + P 331: IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. Products containing Crystalline Silica are Class D2B - Toxic, and containing Calcium Oxide are Class E - WHMIS Corrosive. Classification of substance (Regulation (EC) No 1272/2008): Skin Corrosion/Irritation (Category 1C); Serious eye ECHA damage/Irritation (Category 1) In contrast to it's dry form, calcium oxide, when diluted with water, will release heat and cause skin and eye OTHER damage (alkaline burns) with prolonged contact. -

Cement Kiln and Waste to Energy Incineration of Spent Media Craig Patterson1, Seyed A
Cement Kiln and Waste to Energy Incineration of Spent Media Craig Patterson1, Seyed A. Dastgheib2 1U.S. Environmental Protection Agency, Center for Environmental Solutions and Emergency Response 2Illinois State Geological Survey, University of Illinois at Urbana-Champaign THERMAL TREATMENT OF PFAS STATE OF THE SCIENCE WORKSHOP Sponsored by U.S. Environmental Protection Agency's (EPA) Office of Research and Development (ORD) and Department of Defense SERDP/ESTCP Programs Cincinnati, Ohio February 25, 2020 Project Focus: Incineration of PFAS-laden Ion Exchange Resins with a Lime Sludge Additive PFAS removal from water by ion exchange resins Management of spent PFAS-laden resins Rotary kilns (e.g., cement kilns) for solid waste and waste to energy incineration Incineration of PFAS-laden resin in rotary kilns . HF capture after incineration is needed . Calcium additive can capture fluorine Lime sludge reuse as a low cost additive for fluorine capture during incineration Source: Bernard, B., DiPasquale, M.., From Pilot to Full-Scale: A Case Study for the Treatment of PFC’s with Ion Exchange, AWWA ACE, Denver, CO 2019. 2 PFAS Removal from Water Ion exchange and activated carbon adsorption are identified as the most mature and feasible technologies for PFAS removal Anion exchange resins can be used as a stand-alone treatment or in combination with GAC Anion exchange resins have shown excellent performance for PFAS removal at relatively low EBCTs when compared to GAC Sources: 1) I. Ross et al. A review of merging technologies for remediation of PFASs. Remediation 2018, 28, 101-126. 2) Purolite 3 presentation and case study. F. Boodoo et al. -

White CSA CEMENT Calcium Sulfo Aluminate
® white CSA CEMENT Calcium Sulfo Aluminate An innovation in cement technology for aesthetic and technical applications. is a special Calcium Sulfo Aluminate cement (CSA) designed for decorative concrete, terrazzo, flooring, glass fiber reinforced concrete (GFRC), dry-mixed mortars, architectural precast, fiber cement and more. Specially selected high purity raw materials, optimized calcination and carefully monitored grinding guarantee a consistent white color. Features: • Fast setting • High early strength (1-day strength equals the 28-day strength of ordinary cement) • Continuous strength development (no loss of strength in time) • Rapid drying (C4A3Š autogenously binds excess water) • Shrinkage compensated – (little to NO shrinkage) • Low alkali Benefits: • Ideal for making “ fast set concrete” • Allows for quick-demolding • Rapid return to service • Compatible with various aggregates • Reduces efflorescence Calcium Sulfoaluminate Cement increases strength, reduces set times, and decreases shrinkage of concrete mix designs. ,used as a stand-alone binder or blended with white portland cement delivers high early strength to incredibly durable concrete and mortar. Conventional retarding admixtures can be used to increase working time sacrificing early strength development Special Cement Department CALTRA US.- exclusive distributor; DELTA PERFORMANCE 9126 Industrial Blvd.,Suite B , Covington, GA 30014 T: +1 678 729 9330 F:+1 678 729 9336 M:+1 770 231 2674 W: www.caltra.com ® Calcium Sulfoaluminate Cement is ideal for applications requiring high early strength and rapid setting. Concrete and mortar formulated with CSA cement are capable of obtaining the 28-day strength of ordinary cement in just one day. Suitable projects include: • Concrete runway repair • Bridge deck repair • Tunneling • Roadway repairs • Non-shrink grout • Concrete floor overlayment • Concrete repair mortar Low Carbon Footprint The amount of energy used to produce Calcium Sulfoaluminate Cement is significantly lower than for portlandcement.