Emissions Control of Hydrochloric and Fluorhydric Acid in Cement Factories from Romania
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vibrationally Excited Hydrogen Halides : a Bibliography On
VI NBS SPECIAL PUBLICATION 392 J U.S. DEPARTMENT OF COMMERCE / National Bureau of Standards National Bureau of Standards Bldg. Library, _ E-01 Admin. OCT 1 1981 191023 / oO Vibrationally Excited Hydrogen Halides: A Bibliography on Chemical Kinetics of Chemiexcitation and Energy Transfer Processes (1958 through 1973) QC 100 • 1X57 no. 2te c l !14 c '- — | NATIONAL BUREAU OF STANDARDS The National Bureau of Standards' was established by an act of Congress March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau consists of the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Institute for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and commerce. The Institute consists of a Center for Radiation Research, an Office of Meas- urement Services and the following divisions: Applied Mathematics — Electricity — Mechanics — Heat — Optical Physics — Nuclear Sciences" — Applied Radiation 2 — Quantum Electronics 1 — Electromagnetics 3 — Time 3 1 1 and Frequency — Laboratory Astrophysics — Cryogenics . -
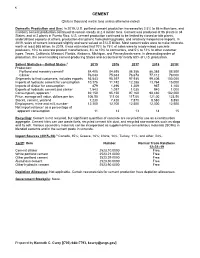
Cement Data Sheet
42 CEMENT (Data in thousand metric tons unless otherwise noted) Domestic Production and Use: In 2019, U.S. portland cement production increased by 2.5% to 86 million tons, and masonry cement production continued to remain steady at 2.4 million tons. Cement was produced at 96 plants in 34 States, and at 2 plants in Puerto Rico. U.S. cement production continued to be limited by closed or idle plants, underutilized capacity at others, production disruptions from plant upgrades, and relatively inexpensive imports. In 2019, sales of cement increased slightly and were valued at $12.5 billion. Most cement sales were to make concrete, worth at least $65 billion. In 2019, it was estimated that 70% to 75% of sales were to ready-mixed concrete producers, 10% to concrete product manufactures, 8% to 10% to contractors, and 5% to 12% to other customer types. Texas, California, Missouri, Florida, Alabama, Michigan, and Pennsylvania were, in descending order of production, the seven leading cement-producing States and accounted for nearly 60% of U.S. production. Salient Statistics—United States:1 2015 2016 2017 2018 2019e Production: Portland and masonry cement2 84,405 84,695 86,356 86,368 88,500 Clinker 76,043 75,633 76,678 77,112 78,000 Shipments to final customers, includes exports 93,543 95,397 97,935 99,406 100,000 Imports of hydraulic cement for consumption 10,376 11,742 12,288 13,764 15,000 Imports of clinker for consumption 879 1,496 1,209 967 1,100 Exports of hydraulic cement and clinker 1,543 1,097 1,035 940 1,000 Consumption, apparent3 92,150 95,150 97,160 98,480 102,000 Price, average mill value, dollars per ton 106.50 111.00 117.00 121.00 123.50 Stocks, cement, yearend 7,230 7,420 7,870 8,580 8,850 Employment, mine and mill, numbere 12,300 12,700 12,500 12,300 12,500 Net import reliance4 as a percentage of apparent consumption 11 13 13 14 15 Recycling: Cement is not recycled, but significant quantities of concrete are recycled for use as a construction aggregate. -

8.7 Hydrofluoric Acid 8.7.1 General
8.7 Hydrofluoric Acid 8.7.1 General5-6 Hydrogen fluoride (HF) is listed as a Title III Hazardous Air Pollutant. Hydrogen fluoride is produced in 2 forms, as anhydrous hydrogen fluoride and as aqueous hydrofluoric acid. The predominant form manufactured is hydrogen fluoride, a colorless liquid or gas that fumes on contact with air and is water soluble. Traditionally, hydrofluoric acid has been used to etch and polish glass. Currently, the largest use for HF is in aluminum production. Other HF uses include uranium processing, petroleum alkylation, and stainless steel pickling. Hydrofluoric acid is also used to produce fluorocarbons used in aerosol sprays and in refrigerants. Although fluorocarbons are heavily regulated due to environmental concerns, other applications for fluorocarbons include manufacturing of resins, solvents, stain removers, surfactants, and pharmaceuticals. 8.7.2 Process Description1-3,6 Hydrofluoric acid is manufactured by the reaction of acid-grade fluorspar (CaF2) with sulfuric acid (H2SO4) as shown below: → CaF2 H2SO4 CaSO4 2HF A typical HF plant is shown schematically in Figure 8.7-1. The endothermic reaction requires 30 to 60 minutes in horizontal rotary kilns externally heated to 200 to 250°C (390 to 480°F). Dry fluorspar ("spar") and a slight excess of sulfuric acid are fed continuously to the front end of a stationary prereactor or directly to the kiln by a screw conveyor. The prereactor mixes the components prior to charging to the rotary kiln. Calcium sulfate (CaSO4) is removed through an air lock at the opposite end of the kiln. The gaseous reaction products—hydrogen fluoride and excess H2SO4 from the primary reaction and silicon tetrafluoride (SiF4), sulfur dioxide (SO2), carbon dioxide (CO2), and water produced in secondary reactions—are removed from the front end of the kiln along with entrained particulate. -

Is There an Acid Strong Enough to Dissolve Glass? – Superacids
ARTICLE Is there an acid strong enough to dissolve glass? – Superacids For anybody who watched cartoons growing up, the word unit is based on how acids behave in water, however as acid probably springs to mind images of gaping holes being very strong acids react extremely violently in water this burnt into the floor by a spill, and liquid that would dissolve scale cannot be used for the pure ‘common’ acids (nitric, anything you drop into it. The reality of the acids you hydrochloric and sulphuric) or anything stronger than them. encounter in schools, and most undergrad university Instead, a different unit, the Hammett acidity function (H0), courses is somewhat underwhelming – sure they will react is often preferred when discussing superacids. with chemicals, but, if handled safely, where’s the drama? A superacid can be defined as any compound with an People don’t realise that these extraordinarily strong acids acidity greater than 100% pure sulphuric acid, which has a do exist, they’re just rarely seen outside of research labs Hammett acidity function (H0) of −12 [1]. Modern definitions due to their extreme potency. These acids are capable of define a superacid as a medium in which the chemical dissolving almost anything – wax, rocks, metals (even potential of protons is higher than it is in pure sulphuric acid platinum), and yes, even glass. [2]. Considering that pure sulphuric acid is highly corrosive, you can be certain that anything more acidic than that is What are Superacids? going to be powerful. What are superacids? Its all in the name – super acids are intensely strong acids. -

[email protected] +1-703-527-3887 (International) Website
Date of Issue: 10 May 2016 SAFETY DATA SHEET 1. SUBSTANCE AND SOURCE IDENTIFICATION Product Identifier SRM Number: 3122 SRM Name: Hafnium (Hf) Standard Solution Other Means of Identification: Not applicable. Recommended Use of This Material and Restrictions of Use This Standard Reference Material (SRM) is intended for use as a primary calibration standard for the quantitative determination of hafnium. A unit of SRM 3122 consists of 50 mL of an acidified aqueous solution prepared gravimetrically to contain a known mass fraction of hafnium in a polyethylene bottle sealed in an aluminized bag. The solution contains volume fractions of nitric acid at approximately 1 % to 10 % and hydrofluoric acid at approximately 1 % to 2 %. Company Information National Institute of Standards and Technology Standard Reference Materials Program 100 Bureau Drive, Stop 2300 Gaithersburg, Maryland 20899-2300 Telephone: 301-975-2200 Emergency Telephone ChemTrec: FAX: 301-948-3730 1-800-424-9300 (North America) E-mail: [email protected] +1-703-527-3887 (International) Website: http://www.nist.gov/srm 2. HAZARDS IDENTIFICATION Classification Physical Hazard: Not classified. Health Hazard: Skin Corrosion/Irritation Category 1B Serious Eye Damage/Eye Irritation Category 1 Label Elements Symbol Signal Word DANGER Hazard Statement(s) H314 Causes severe skin burns and eye damage. Precautionary Statement(s) P260 Do not breathe fumes, mists, vapors, or spray. P264 Wash hands thoroughly after handling. P280 Wear protective gloves, protective clothing, and eye protection. P301+P330+P331 If swallowed: Rinse mouth. Do NOT induce vomiting. P303+P361+P353 If on skin (or hair): Remove immediately all contaminated clothing. Rinse skin with water. -

Questions ALKENES: REACTIONS with HYDROGEN HALIDES
Chemguide – questions ALKENES: REACTIONS WITH HYDROGEN HALIDES 1. State Markovnikov's Rule. 2. Show the structural formulae for the main product of each of the following addition reactions between various alkenes and a hydrogen halide. (This question is also testing your ability to write the structures for alkenes given their names. If you can't do that, get it sorted out before you continue!) a) ethene and hydrogen bromide b) but-2-ene and hydrogen chloride c) but-1-ene and hydrogen chloride d) propene and hydrogen iodide e) 2-methylbut-2-ene and hydrogen iodide f) but-1-ene and hydrogen bromide where everything is pure g) but-1-ene and hydrogen bromide in the presence of oxygen or organic peroxides 3. a) How does the rate of the reaction change as you go along the series HF – HCl – HBr – HI? b) Briefly explain the trend you have given in part (a). c) How does the rate of reaction change as you go from ethene to propene to 2-methylbut-2-ene? d) Alkyl groups (like methyl and ethyl groups) have a tendency to “push” electrons away from themselves towards the double bond. (i) How does this help to explain the way the attractiveness of the double bond varies from ethene to propene to 2-methylbut-2-ene? (ii) The mechanism for the reactions involves the formation of intermediate ions with the positive charge on a carbon atom (carbocations). The intermediate carbocations in these cases would be + + + CH CH CH CHCH CH CCH CH 3 2 3 3 3 2 3 CH3 Why does this help to explain the variation in reactivity that you should have given in part (c)? www.chemguide.co.uk. -

Chlorine and Hydrogen Chloride
This report contains the collective views of an nternational group of experts and does not xcessarily represent the decisions or the stated 1 icy of the United Nations Environment Pro- '€mme, the International Labour Organisation, or the World Health Organization. Environmental Health Criteria 21 CHLORINE AND HYDROGEN CHLORIDE 'ublished under the joint sponsorship of Ic United Nations Environment Programme. the International Labour Organisation, and the World Health Organization / \r4 ( o 4 UI o 1 o 'T F- World Health Organization kz Geneva, 1982 The International Programme on Chemical Safety (IPCS) is a joint ven- ture of the United Nations Environment Programme. the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the environment. Supporting activities include the development of epidemiological, experi- mental laboratory, and risk assessment methods that could produce interna- tionally comparable results, and the development of manpower in the field of toxicology. Other relevant activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination of laboratory testing and epidemiological studies, and promotion of research on the mechanisms of the biological action of chemicals. ISBN 92 4 154081 8 World Health Organization 1982 Publications of the World Health Organization enjoy copyright protec- tion in accordance with the provisions of Protocol 2 of the Universal Copy- right Convention. For rights of reproduction or translation of WHO publica- tions, in part or in loto, application should be made to the Office of Publica- tions, World Health Organization, Geneva. Switzerland. The World Health Organization welcomes such applications. -

Method for Removing Impurities from Anhydrous Hydrogen Chloride
Europaisches Patentamt 0 380 014 J European Patent Office (iO Publication number: A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 90101184.1 (?) int.ci.5:C01B 7/07 @ Date of filing: 22.01.90 © Priority: 27.01.89 US 303513 © Applicant: THE DOW CHEMICAL COMPANY P.O. Box 1967 © Date of publication of application: Midland, Michigan 48641 -1967(US) 01.08.90 Bulletin 90/31 © Inventor: Repman, Josef F. © Designated Contracting States: 31 Wagon Lane Loop BE DE ES FR GB IT NL Angelton, Texas 7751 5(US) Inventor: Morris, Thomas E. 324 Linden Lane Lake Jackson, Texas 77566(US) Inventor: Hill, Thomas F. 128 Lily Lake Jackson, Texas 77566(US) Representative: Sternagel, Hans-Gunther, Dr. etal Patentanwalte Dr. Michael Hann Dr. H.-G. Sternagel Sander Aue 30 D-5060 Bergisch Gladbach 2(DE) © Method for removing impurities from anhydrous hydrogen chloride. © A method to purify impure gaseous hydrogen chloride containing unsaturated chlorinated hydrocarbons in an amount less than that necessary to inhibit purification comprising exposing the impure hydrogen chloride to an ultraviolet light source in the presence of gaseous chlorine for a sufficient time for the gaseous chlorine to react with organic impurities in the hydrogen chloride to form heavier organic compounds and thereafter separating the heavier compounds from the hydrogen chloride. < O 00 CO a. LU Xerox Copy Centre EP 0 380 014 A1 METHOD FOR REMOVING IMPURITIES FROM ANHYDROUS HYDROGEN CHLORIDE This invention pertains to hydrogen chloride and more in particular to a method to remove impurities from anhydrous hydrogen chloride. Hydrogen chloride is produced as a byproduct in many chemical processes. -

Use of Cement Kiln Dust for Subgrade Stabilization
Report No. KS-04-3 FINAL REPORT USE OF CEMENT KILN DUST FOR SUBGRADE STABILIZATION Robert L. Parsons, Ph.D., P.E. Elizabeth Kneebone University of Kansas Justin P. Milburn Continental Consulting Engineers, Inc. OCTOBER 2004 KANSAS DEPARTMENT OF TRANSPORTATION Division of Operations Bureau of Materials and Research 1 Report No. 2 Government Accession No. 3 Recipient Catalog No. KS-04-3 4 Title and Subtitle 5 Report Date USE OF CEMENT KILN DUST FOR SUBGRADE STABILIZATION October 2004 6 Performing Organization Code 7 Author(s) 8 Performing Organization Report Robert L. Parsons, Ph.D., P.E., Elizabeth Keebone, both of University of No. Kansas, and Justin P. Milburn, Continental Consulting Engineers, Inc. 9 Performing Organization Name and Address 10 Work Unit No. (TRAIS) University of Kansas 1530 West 15th Street; Learned Hall 11 Contract or Grant No. Lawrence, Kansas 66045-7609 C1378 12 Sponsoring Agency Name and Address 13 Type of Report and Period Kansas Department of Transportation Covered Bureau of Materials and Research Final Report 700 SW Harrison Street October 2002 to July 2004 Topeka, Kansas 66603-3754 14 Sponsoring Agency Code RE-0331-01 15 Supplementary Notes Fly Ash Management. L.L.C., of Topeka, Kansas also provided funding for this project. For more information on this report, please write to address in block 9. 16 Abstract Poor subgrade soil conditions can result in inadequate pavement support and reduce pavement life. Soils may be improved through the addition of chemical or cementitious additives. These chemical additives range from waste products to manufactured materials and include lime, Class C fly ash, Portland cement, cement kiln dust from pre-calciner and long kiln processes, and proprietary chemical stabilizers. -

Carbon Dioxide Control Technologies for the Cement Industry Volker Hoenig, Düsseldorf, Germany
Carbon Dioxide Control Technologies for the Cement Industry Volker Hoenig, Düsseldorf, Germany GCEP Workshop “Carbon Management in Manufacturing Industries” Stanford University, 15/16 April 2008 Carbon Dioxide Control Technologies for the Cement Industry 1. Introduction 2. The cement clinker burning process 3. Assessment of carbon dioxide control technologies 3.1 Pre-combustion technologies 3.2 Oxyfuel technology 3.3 Post-combustion technologies 4. Preliminary research results (Oxyfuel technology) 4.1 Impact on raw meal decarbonation 4.2 Modeling of the clinker burning process with Oxyfuel operation The German Cement Works Association (VDZ) Activities: • Mortar and concrete • Chemistry and mineralogy • Environmental expertise • Plant technology investigations • Environmental measurements • Certification • Knowledge transfer ↑ The Research Institute of the Cement Industry, Düsseldorf / Germany For further information: www.vdz-online.de The European Cement Research Academy (ECRA) • has been founded by VDZ in 2004 • Members (> 40) are cement companies and associations from Europe, Asia, Australia, US • objectives Æ know how transfer Æ joint research • activities Æ seminars and workshops Æ research programmes - carbon capture technologies for the cement industry - continuous measurement of biomass CO2 in stack gases For further information: www.ecra-online.org Carbon Dioxide Control Technologies for the Cement Industry 1. Introduction 2. The cement clinker burning process 3. Assessment of carbon dioxide control technologies 3.1 Pre-combustion -

Reactions of Alkenes and Alkynes
05 Reactions of Alkenes and Alkynes Polyethylene is the most widely used plastic, making up items such as packing foam, plastic bottles, and plastic utensils (top: © Jon Larson/iStockphoto; middle: GNL Media/Digital Vision/Getty Images, Inc.; bottom: © Lakhesis/iStockphoto). Inset: A model of ethylene. KEY QUESTIONS 5.1 What Are the Characteristic Reactions of Alkenes? 5.8 How Can Alkynes Be Reduced to Alkenes and 5.2 What Is a Reaction Mechanism? Alkanes? 5.3 What Are the Mechanisms of Electrophilic Additions HOW TO to Alkenes? 5.1 How to Draw Mechanisms 5.4 What Are Carbocation Rearrangements? 5.5 What Is Hydroboration–Oxidation of an Alkene? CHEMICAL CONNECTIONS 5.6 How Can an Alkene Be Reduced to an Alkane? 5A Catalytic Cracking and the Importance of Alkenes 5.7 How Can an Acetylide Anion Be Used to Create a New Carbon–Carbon Bond? IN THIS CHAPTER, we begin our systematic study of organic reactions and their mecha- nisms. Reaction mechanisms are step-by-step descriptions of how reactions proceed and are one of the most important unifying concepts in organic chemistry. We use the reactions of alkenes as the vehicle to introduce this concept. 129 130 CHAPTER 5 Reactions of Alkenes and Alkynes 5.1 What Are the Characteristic Reactions of Alkenes? The most characteristic reaction of alkenes is addition to the carbon–carbon double bond in such a way that the pi bond is broken and, in its place, sigma bonds are formed to two new atoms or groups of atoms. Several examples of reactions at the carbon–carbon double bond are shown in Table 5.1, along with the descriptive name(s) associated with each. -

HYDROGEN FLUORIDE Safety Data Sheet
Revision Date 14-May-2015 , Version 1 _________________________________________________________________________________ HYDROGEN FLUORIDE Safety Data Sheet _________________________________________________________________________________ 1. IDENTIFICATION Product identifier Product Name HYDROGEN FLUORIDE Other means of identification Safety data sheet number LIND-P070 UN/ID no. UN1052 Synonyms Hydrofluoric acid, anhydrous Recommended use of the chemical and restrictions on use Recommended Use Industrial and professional use. Uses advised against Consumer use Details of the supplier of the safety data sheet Linde Gas North America LLC - Linde Merchant Production Inc. - Linde LLC 575 Mountain Ave. Murray Hill, NJ 07974 Phone: 908-464-8100 www.lindeus.com Linde Gas Puerto Rico, Inc. Road 869, Km 1.8 Barrio Palmas, Catano, PR 00962 Phone: 787-641-7445 www.pr.lindegas.com Linde Canada Limited 5860 Chedworth Way Mississauga, Ontario L5R 0A2 Phone: 905-501-1700 www.lindecanada.com * May include subsidiaries or affiliate companies/divisions. For additional product information contact your local customer service. Emergency telephone number Company Phone Number 800-232-4726 (Linde National Operations Center, US) 905-501-0802 (Canada) CHEMTREC: 1-800-424-9300 (North America) +1-703-527-3887 (International) 2. HAZARDS IDENTIFICATION _____________________________________________________________________________________________ Page 1 / 11 LIND-P070 HYDROGEN FLUORIDE Revision Date 14-May-2015 _____________________________________________________________________________________________