RESEARCH ARTICLE Independently Evolved Upper Jaw Protrusion Mechanisms Show Convergent Hydrodynamic Function in Teleost Fishes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Order ZEIFORMES PARAZENIDAE Parazens P.C
click for previous page Zeiformes: Parazenidae 1203 Order ZEIFORMES PARAZENIDAE Parazens P.C. Heemstra, South African Institute for Aquatic Biodiversity, South Africa iagnostic characters: Small to moderate-sized (to 30 cm) oblong fishes, the head and body com- Dpressed; body depth slightly less than head length, contained 2.6 to 2.9 times in standard length; head naked, the bones thin and soft; opercular bones weakly serrate; mouth large, terminal, the upper jaw extremely protrusile; maxilla widely expanded posteriorly, and mostly exposed when mouth is closed; no supramaxilla; jaws with 1 or 2 rows of small, slender, conical teeth; vomer with a few short stout teeth;gill rakers (including rudiments) 2 on upper limb, 8 on lower limb.Eye diameter about 1/3 head length and slightly less than snout length.Branchiostegal rays 7.Dorsal fin divided, with 8 slender spines and 26 to 30 soft rays; anal fin with 1 minute spine and 30 to 32 soft rays; dorsal-, anal-, and pectoral-fin rays un- branched; caudal fin forked, with 11 principal rays and 9 branched rays; pectoral fin with 15 or 16 rays, shorter than eye diameter; pelvic fins with 1 unbranched and 5 or 6 branched soft rays, but no spine, fin origin posterior to a vertical at pectoral-fin base. Scales moderate in size, weakly ctenoid, and deciduous; 2 lateral lines originating on body at upper end of operculum and running posteriorly about 4 scale rows apart, gradually converging to form a single line on caudal peduncle. Caudal peduncle stout, the least depth about equal to its length and slightly less than eye diameter.Vertebrae 34.Colour: body reddish or silvery; large black blotch on anterior margin of dorsal fin. -

Percomorph Phylogeny: a Survey of Acanthomorphs and a New Proposal
BULLETIN OF MARINE SCIENCE, 52(1): 554-626, 1993 PERCOMORPH PHYLOGENY: A SURVEY OF ACANTHOMORPHS AND A NEW PROPOSAL G. David Johnson and Colin Patterson ABSTRACT The interrelationships of acanthomorph fishes are reviewed. We recognize seven mono- phyletic terminal taxa among acanthomorphs: Lampridiformes, Polymixiiformes, Paracan- thopterygii, Stephanoberyciformes, Beryciformes, Zeiformes, and a new taxon named Smeg- mamorpha. The Percomorpha, as currently constituted, are polyphyletic, and the Perciformes are probably paraphyletic. The smegmamorphs comprise five subgroups: Synbranchiformes (Synbranchoidei and Mastacembeloidei), Mugilomorpha (Mugiloidei), Elassomatidae (Elas- soma), Gasterosteiformes, and Atherinomorpha. Monophyly of Lampridiformes is justified elsewhere; we have found no new characters to substantiate the monophyly of Polymixi- iformes (which is not in doubt) or Paracanthopterygii. Stephanoberyciformes uniquely share a modification of the extrascapular, and Beryciformes a modification of the anterior part of the supraorbital and infraorbital sensory canals, here named Jakubowski's organ. Our Zei- formes excludes the Caproidae, and characters are proposed to justify the monophyly of the group in that restricted sense. The Smegmamorpha are thought to be monophyletic principally because of the configuration of the first vertebra and its intermuscular bone. Within the Smegmamorpha, the Atherinomorpha and Mugilomorpha are shown to be monophyletic elsewhere. Our Gasterosteiformes includes the syngnathoids and the Pegasiformes -

Order Myctophiformes, Lanternfishes
Order Myctophiformes, lanternfishes • 241 species, 35 genera, 2 families • Deep sea pelagic and benthic, numerically dominant in deep sea habitats • Large terminal mouth (reminiscent of anchovy) • Adipose fin present • Compressed head and body (Myctophiformes = nose serpent shape) Lampridiformes • Large eyes Percopsiformes • Photophores Acanthomorpha •Hollow unsegmented spines on dorsal and anal fins •Rostal and premaxilla cartilidge and ligaments allow greater jaw protrusability Order Lampridiformes, opahs and oarfish Order Lampridiformes, opahs and oarfish • Oarfish • 19 species, 12 genera, 7 families – Longest teleost – over 30 feet • no true spines in fins – Only one individual observed • unique upper jaw protrusion – alive, used amiiform maxilla not directly attached to swimming ethmoid or palentine • deep bodied or ribbon-like • pelagic and deep water marine 1 Order Percopsiformes, trout perch, pirate perch, cavefish • 3 families, 7 genera, 9 species • All freshwater • Few with adipose fins – one of the most derived fishes with them • Pirate perch (Aphredoderidae) – One species – Fairly extensive parental care – Anus migration • Cavefish (Amblyopsidae) Zeiformes – Reduction or loss of eyes Gadiformes – Sensory papillae on head, body and tail Acanthomorpha •Hollow unsegmented spines on – Anus migration dorsal and anal fins •Rostal and premaxilla cartilidge – Convergent evolution of cave fish and ligaments allow greater jaw and other cave characins, protrusability catfishes etc. Order Zeiformes Order Gadiiformes • Dories • 555 species, -
Field Identification Guide to the Living Marine Resources in Kenya
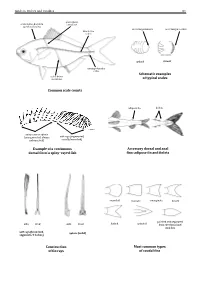
Guide to Orders and Families 81 lateral line scales above scales before dorsal fin outer margin smooth outer margin toothed (predorsal scales) lateral–line 114 scales cycloid ctenoidِّ scales circumpeduncular Schematic examples lateral line of typical scales scales below Common scale counts adipose fin finlets soft rays (segmented, spinyunbranched) rays or spines usually branched) (unsegmented, always Example of a continuous Accessory dorsal and anal dorsal fin of a spiny–rayed fish fins: adipose fin and finlets rounded truncate emarginate lunate side front side front from the dorsal and pointed and separated forked pointed soft rays (branched, spines (solid) segments, 2 halves) anal fins Construction Most common types of fin rays of caudal fins 82 Bony Fishes GUIDE TO ORDERS AND FAMILIES Order ELOPIFORMES – Tarpons and allies Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 23–25 branchiostegal rays; upper jaw extending past eye; tip of snout not overhanging mouth; colour silvery. ELOPIDAE Page 121 very small scales Ladyfishes To 90 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head large mouth gular plate MEGALOPIDAE Page 121 last ray long Tarpons large scales To 55 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head gular plate Order ALBULIFORMES – Bonefishes Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 6–16 branchiostegal rays; upper jaw not extending as far as front of eye; tip of snout overhanging mouth; colour silvery. -

Randal Singer, Ph.D
CURRICULUM VITAE Randal Singer, Ph.D. The University of Michigan Email: [email protected]; Tel: (352) 209-1024 Office: 1421 Research Museums Center, 3600 Varsity Drive, Ann Arbor, MI 48108-2228 Website: https://randchovy.wixsite.com/randalsinger EDUCATION 2019 PhD, Interdisciplinary Ecology The University of Florida/iDigBio, Gainesville, FL Thesis advisor: Lawrence M. Page Dissertation: An Interdisciplinary Approach to Increasing the Sustainability of Biodiversity Collections 2013 M.S., Zoology The University of Florida, Gainesville, FL Advisor: Lawrence M. Page Thesis: A Revision of the genus Acanthocobitis (Peters 1861) with description of a new genus: Paracanthocobitis (Teleostei: Nemacheilidae) 2008 B.S., Ecology The University of Georgia, Athens, GA Senior thesis: Tracing carbon flow in deep-sea whale-fall communities using STELLA. PROFESSIONAL APPOINTMENTS/EMPLOYMENT 2019-Present The University of Michigan– Asst. Research Scientist/Collection Manager, Fishes Management, curation, and coordination of collection resources and activities Facilitation of collections based research, education and outreach Aiding research uses of collection through visits and external loans Personal scientific research on fishes (734) 936-3754 2015-2019 The University of Florida/iDigBio – PhD Candidate Conducting informatics research using specimen data Advocating for collections care and use Organizing workshops and webinars for iDigBio Representing iDigBio at conferences and events (352) 273-1374 2013-2015 University of Alabama – Collections Manager, Ichthyology Responsible for specimen cataloguing and curation Oversee all activities of the fish and herpetology collections Conduct and aid in research both in and outside of the collection Aiding in public outreach and specimen loans for research (205) 348-1822 2010-2013 Florida Museum of Natural History – Asst. -

Diet Composition and Feeding Strategy of John Dory, Zeus Faber, in the Coastal Waters of Korea Han Ju Kim1, Hyeong-Gi Kim2 and Chul-Woong Oh1*
Kim et al. Journal of Ecology and Environment (2020) 44:8 Journal of Ecology https://doi.org/10.1186/s41610-020-00153-y and Environment SHORT COMMUNICATION Open Access Diet composition and feeding strategy of John Dory, Zeus faber, in the coastal waters of Korea Han Ju Kim1, Hyeong-Gi Kim2 and Chul-Woong Oh1* Abstract Background: Most fish undergo prey switch from juvenile to adult. It is thought that slightly different feeding habits occur among adult fishes due to growth, spawning, habitat change, and so on. Therefore, the diet of the John Dory Zeus faber (≥ 24 cm TL) was studied in the coastal waters of Korea by analysis of stomach contents, with comparison by season and size class of diet composition and prey diversity. Monthly samples were taken from February 2017 to January 2018. Results: The results showed that the John Dory was a piscivorous predator, and pisces had occupied 82.3% of IRI%. Trichiurus lepturus and Trachurus japonicus were important preys in all size classes and seasons. Diet composition differed among the size classes and seasons (Chi-square test, P < 0.05). As body size of Z. faber increased, the occurrence of benthic fish (Glyptocephalus stelleri) tended to increase. The seasonal prey composition also changed depending on the abundant species of each season. Conclusions: Z. faber is a piscivorous predator. The consumption habits of Z. faber appear to different results by their size and seasons. This study suggests that Z. faber could be considered an opportunistic predator. Keywords: John Dory, Zeus faber, Diet composition, Feeding habits, Feeding ecology Background Aegean Sea (Ismen et al. -

Seven “Super Orders” • 7) Superorder Acanthopterygii • ‘Three ‘Series’ • 1) Mugilomorpha – Mullets – 66 Species, Economically Important; Leap from Water.??
• Superorder Acanthopterygii • Mugilomorpha – Order Mugiliformes: Mullets • Atherinomorpha ACANTHOPTERYGII = Mugilomorpha (mullets) + – Order Atheriniformes: Silversides and rainbowfishes Atherinomorpha (silversides) + Percomorpha – Order Beloniformes: Needlefish, Halfbeaks, and Flyingfishes – Order Cyprinodontiformes: Killifishes,plays, swordtails + ricefishes (Medaka) • Series Percomorpha – ?Order Stephanoberciformes: Pricklefish, whalefish – ?Order Bercyformes: Squirrelfishes, redfishes, Pineapple fishes, flashlight fishes, Roughies, Spinyfins, Fangtooths – ?Order Zeiformes: Dories, Oreos, . – Order Gasterosteiformes: Pipefish and seahorses, sticklebacks – Order Synbranchiformes: Swampeels – Order Scorpaeniformes: Scorpianfish – Order Perciformes: Many many – Order Pleuronectiformes: Flounders and soles – Order Tetraodontiformes: Triggers and puffers etc Acanthopterygii Phylogeny – Johnson and Wiley Seven “Super Orders” • 7) Superorder Acanthopterygii • ‘Three ‘Series’ • 1) Mugilomorpha – mullets – 66 species, economically important; leap from water.?? 1 Seven “Super Orders” Seven “Super Orders” • 7) Superorder Acanthopterygii - 3 ‘Series’ • 2) Atherinomorpha – Surface of water. • 7) Superorder Acanthopterygii – 13,500 • a) Atheriniformes - silversides, rainbow fish, species in 251 families. 285 spp.; • b) Beloniformes = needlefishes, flying fishes, include Medakas (ricefish Oryzias - used in 3) Percomorpha 12,000 species with labs; first to have sex in space); and anteriorly placed pelvic girdle that is • c) Cyprinodontiformes = Poeciliids -

Direct Identification of Fish Species by Surface Molecular Transferring
Electronic Supplementary Material (ESI) for Analyst. This journal is © The Royal Society of Chemistry 2020 Supplementary materials for Direct Identification of Fish Species by Surface Molecular Transferring Mingke Shao, Hongyan Bi* College of Food Science and Engineering, Shanghai Ocean University, Hucheng Ring Road 999, Pudong New District, 201306 Shanghai, China * To whom correspondence should be addressed. E-mail address: [email protected] E-mail address for the other authors: [email protected] S1 S1. Photos and information on the analyzed fish samples Fig. S1. Photos of fishes analyzed in the present study: (A) Oreochromis mossambicus (B) Epinephelus rivulatus (C) Mugil cephalus; (D) Zeus faber (E) Trachinotus ovatus (F) Brama japonica (G) Larimichthys crocea (H) Larimichthys polyactis (I) Pampus argenteus. Scale bar in each photo represents 1 cm. Table S1. List of the scientific classification of fishes analyzed in the study. The classification of fishes refers to https://www.fishbase.de/. Binomial Abbreviatio English Chinese name n common Scientific classification name (Scientific name name) Actinopterygii (class) > Perciformes (order) > Japanese Brama Brama BJ Bramidae (family) > Wufang japonica japonica Brama (genus) > B. brama (species) Actinopterygii (class) > Silver Scombriformes(order) > Baichang pomfret; Pampus PA ( Fish White argenteus Stromateidae family) > pomfret Pampus (genus) > P. argenteus (species) Haifang Zeus faber Actinopterygii (class) > (commonly Linnaeu; Zeus faber ZF Zeiformes (order) > called: John Dory; Zeidae (family) > S2 Yueliang target perch Zeus (genus) > Fish) Z. faber (species) Actinopteri (class) > OM Cichliformes (order) > Mozambique Oreochromis Cichlidae (family) > Luofei Fish tilapia mossambicus Oreochromis (genus) > O.mossambicus (species) Actinopterygii (class) > MC Mugiliformes (order) Xiaozhai Flathead Mugil Mugilidae (family) > Fish grey mullet cephalus Mugil (genus) > M. -

A New Species of Zenopsis (Zeiformes: Zeidae) from the South China Sea, East China Sea and Off Western Australia
Memoirs of Museum Victoria 63(1): 91–96 (2006) ISSN 1447-2546 (Print) 1447-2554 (On-line) http://www.museum.vic.gov.au/memoirs/index.asp A new species of Zenopsis (Zeiformes: Zeidae) from the South China Sea, East China Sea and off Western Australia TETSUJI NAKABO1, DIANNE J. BRAY2 AND UMEYOSHI YAMADA3 1Kyoto University Museum, Kyoto University, Kyoto 606-8501, Japan ([email protected]) 2Museum Victoria, GPO Box 666, Melbourne, Victoria 3001, Australia ([email protected]) 3 Hamada, Togitsu, Nishisonogi, Nagasaki Prefecture 851-2102, Japan Abstract Nakabo T., Bray D.J. and Yamada U. 2006. A new species of Zenopsis (Zeiformes: Zeidae) from the South China Sea, East China Sea and off Western Australia. Memoirs of Museum Victoria 63(1): 91–96. Zenopsis stabilispinosa sp. nov. is described from the South China Sea, East China Sea and off Western Australia. It differs from other species of Zenopsis in having the third anal-fi n spine fused to its pterygiophore, seven dorsal-fi n spines, a short pelvic fi n in adults, and 32 (13+19) vertebrae. A key to the species of Zenopsis is provided. Keywords Zeidae, Zenopsis, new species, South China Sea, Western Australia Introduction Material examined. Holotype. FAKU 64803 (307.2 mm SL), South China Sea, 19º45.0'N, 114º04.0'E, 457–767 m, JAMARC (Japan The species of Zenopsis are characterized by having a deep Marine Fishery Resource Research Center), 20 Jun, 1991. strongly compressed silvery body, scales absent from the body Paratypes. FAKU 64804 (410.1 mm SL), South China Sea, except along the lateral line, concave dorsal head profi le, 19º47.0'N, 114º05.0'E, 465–505 m, JAMARC, 21 Jun 1991; AMS enlarged bucklers along most of the spinous dorsal-fi n base and I.22826-004 (206.1 mm SL), North-west Shelf, Western Australia 210 along the soft dorsal- and anal-fi n bases, three anal-fi n spines, km NW of Port Hedland, 18º44'S, 117º02'E, 396–406 m, J. -

View/Download
ZEIFORMES (Dories) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 5.0 - 28 Sept. 2020 Series ZEIOGADARIA -arius, pertaining to: combination of the subseries Zeiariae (from Zeiformes) and Gadariae (from Gadiformes) Subseries ZEIARIAE Order ZEIFORMES Dories 6 families · 16 genera · 34 species Suborder CYTTOIDEI Family CYTTIDAE Lookdown Dories Cyttus Günther 1860 from kittos, Greek name for the ivy plant, inexplicably inserted into a list of fishes by Greco-Egyptian author Athenaeus (late 2nd to early 3rd centuries AD), which Günther, believing it to be the name of an unknown fish, applied to this genus Cyttus australis (Richardson 1843) southern, referring to distribution in the southern hemisphere around Australia Cyttus novaezealandiae (Arthur 1885) of New Zealand, referring to type locality off Otago Heads, New Zealand (also occurs in the southwest Pacific off Australia) Cyttus traversi Hutton 1872 in honor of New Zealand politician, lawyer, explorer and naturalist William Thomas Locke Travers (1819-1903), who “presented” type Suborder ZEIODEI Family OREOSOMATIDAE Oreos 4 genera · 10 species Subfamily Pseudocyttinae Pseudocyttus Gilchrist 1906 pseudo-, false, presumed to be “closely related” to Cyttosoma (=Oreosoma) Pseudocyttus maculatus Gilchrist 1906 spotted, referring to large dark spots on gray body Subfamily Oreosomatinae Allocyttus McCulloch 1914 allo-, other, i.e., presumed to be another genus closely related to Cyttosoma (=Oreosoma) Allocyttus folletti Myers 1960 in honor of William I. Follett -

Comprehensive Phylogeny of Ray-Finned Fishes (Actinopterygii) Based on Transcriptomic and Genomic Data
Comprehensive phylogeny of ray-finned fishes (Actinopterygii) based on transcriptomic and genomic data Lily C. Hughesa,b,1,2, Guillermo Ortía,b,1,2, Yu Huangc,d,1, Ying Sunc,e,1, Carole C. Baldwinb, Andrew W. Thompsona,b, Dahiana Arcilaa,b, Ricardo Betancur-R.b,f, Chenhong Lig, Leandro Beckerh, Nicolás Bellorah, Xiaomeng Zhaoc,d, Xiaofeng Lic,d, Min Wangc, Chao Fangd, Bing Xiec, Zhuocheng Zhoui, Hai Huangj, Songlin Chenk, Byrappa Venkateshl,2, and Qiong Shic,d,2 aDepartment of Biological Sciences, The George Washington University, Washington, DC 20052; bNational Museum of Natural History, Smithsonian Institution, Washington, DC 20560; cShenzhen Key Lab of Marine Genomics, Guangdong Provincial Key Lab of Molecular Breeding in Marine Economic Animals, Beijing Genomics Institute Academy of Marine Sciences, Beijing Genomics Institute Marine, Beijing Genomics Institute, 518083 Shenzhen, China; dBeijing Genomics Institute Education Center, University of Chinese Academy of Sciences, 518083 Shenzhen, China; eChina National GeneBank, Beijing Genomics Institute-Shenzhen, 518120 Shenzhen, China; fDepartment of Biology, University of Puerto Rico–Rio Piedras, San Juan 00931, Puerto Rico; gKey Laboratory of Exploration and Utilization of Aquatic Genetic Resources, Shanghai Ocean University, Ministry of Education, 201306 Shanghai, China; hLaboratorio de Ictiología y Acuicultura Experimental, Universidad Nacional del Comahue–CONICET, 8400 Bariloche, Argentina; iProfessional Committee of Native Aquatic Organisms and Water Ecosystem, China Fisheries Association, 100125 Beijing, China; jCollege of Life Science and Ecology, Hainan Tropical Ocean University, 572022 Sanya, China; kYellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, 266071 Qingdao, China; and lComparative Genomics Laboratory, Institute of Molecular and Cell Biology, A*STAR, Biopolis, 138673 Singapore Edited by Scott V.