Development of a Highly Sensitive, Quantitative, and Rapid Detection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
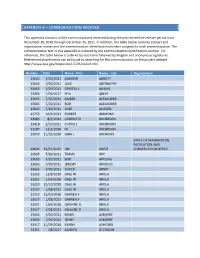
Appendix B – Communications Received
APPENDIX B – COMMUNICATIONS RECEIVED This appendix contains all the communications received during the post-centerline release period from November 18, 2010 through December 31, 2011. In addition, the table below contains contact and organization names and the communication identification number assigned to each communication. The communication text in this appendix is ordered by the communication identification number. For reference, the table below is ordered by last name followed by illegible and anonymous signatures. Referenced attachments can be found by searching for the communication on the project website http://www.bpa.gov/corporate/I-5-EIS/search.cfm. Number Date Name - First Name - Last Organization 13665 7/20/2011 ANDREW ABBOTT 13665 7/20/2011 JACK ABERNATHY 13665 7/20/2011 CRYSTAL L ADAMS 13395 1/29/2011 PHIL AKELY 13667 7/20/2011 AMBER ALEXANDER 13665 7/20/2011 BOB ALEXANDER 13665 7/20/2011 CHEE ALLISON 13755 10/6/2011 ROBERT AMMONS 13683 8/3/2011 CANDICE D ANDERSON 13418 2/10/2011 CURTIS L ANDERSON 13207 12/2/2010 M. ANDERSON 13073 11/22/2010 GINA L ANDREWS STATE OF WASHINGTON, RECREATION AND 13836 12/15/2011 JIM ANEST CONSERVATION OFFICE 13665 7/20/2011 TRAVIS APP 13665 7/20/2011 BOB APPLING 13665 7/20/2011 JEREMY ARIONUS 13665 7/20/2011 CHUCK ARNST 13322 12/8/2010 DALE W AROLA 13321 12/9/2010 DALE W AROLA 13320 12/10/2010 DALE W AROLA 13527 1/28/2011 DALE W AROLA 13320 12/10/2010 DARREN F AROLA 13527 1/28/2011 DARREN F AROLA 13321 12/9/2010 DWAYNE D AROLA 13527 1/28/2011 DWAYNE D AROLA 13665 7/20/2011 BRIAN ASBURRY 13665 -

Organizational Forms in Professional Cycling – Efficiency Issues of the UCI Pro Tour
Organizational Forms in Professional Cycling – Efficiency Issues of the UCI Pro Tour Luca Rebeggiani§ * Davide Tondani DISCUSSION PAPER NO. 345 First Version: August 2006 This Version: July 2007 ISSN: 0949–9962 ABSTRACT: This paper gives a first economic approach to pro cycling and analyses the changes induced by the newly introduced UCI Pro Tour on the racing teams’ behaviour. We develop an oligopolistic model starting from the well known Cournot framework to analyse if the actual setting of the UCI Pro Tour leads to a partially unmeant behaviour of the racing teams. In particular, we show that the blamed regional concentration of their race participation depends on a lack of incentives stemming from the licence assignation procedure. Our theo- retical results are supported by empirical data concerning the performance of the racing teams in 2005 and 2006. As a recommendation for future improvements, we derive from the model the need for a relegation system for racing teams. ZUSAMMENFASSUNG: Der Aufsatz stellt die erste ökonomische Analyse des professionellen Radsports dar. Er analysiert insbesondere die Anreizwirkungen der neuen UCI Pro Tour auf Teams und Fahrer. Ausgehend von dem bekannten Cournot-Ansatz entwickeln wir ein einfaches Oligopol-Modell, um zu untersuchen, ob die der- zeitige Pro Tour-Organisation zu einem unerwünschten Verhalten der Teilnehmer führt. Wir zeigen, dass insbe- sondere das Problem der geographischen Konzentration der Rennteilnahmen der Teams von den mangelnden Anreizen abhängt, die vom jetzigen Lizenzvergabesystem ausgehen. Unsere theoretischen Ergebnisse werden durch empirische Daten aus der Pro Tour 2005 und 2006 gestützt. Als Empfehlung für zukünftige Entwicklun- gen leiten wir aus dem Modell die Notwendigkeit einer Öffnung der Pro Tour ab, mit Auf- und Abstiegsmög- lichkeiten für Rennteams. -
A Genealogy of Top Level Cycling Teams 1984-2016
This is a work in progress. Any feedback or corrections A GENEALOGY OF TOP LEVEL CYCLING TEAMS 1984-2016 Contact me on twitter @dimspace or email [email protected] This graphic attempts to trace the lineage of top level cycling teams that have competed in a Grand Tour since 1985. Teams are grouped by country, and then linked Based on movement of sponsors or team management. Will also include non-gt teams where they are “related” to GT participants. Note: Due to the large amount of conflicting information their will be errors. If you can contribute in any way, please contact me. Notes: 1986 saw a Polish National, and Soviet National team in the Vuelta Espana, and 1985 a Soviet Team in the Vuelta Graphics by DIM @dimspace Web, Updates and Sources: Velorooms.com/index.php?page=cyclinggenealogy REV 2.1.7 1984 added. Fagor (Spain) Mercier (France) Samoanotta Campagnolo (Italy) 1963 1964 1965 1966 1967 1968 1969 1970 1971 1972 1973 1974 1975 1976 1977 1978 1979 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 Le Groupement Formed in January 1995, the team folded before the Tour de France, Their spot being given to AKI. Mosoca Agrigel-La Creuse-Fenioux Agrigel only existed for one season riding the 1996 Tour de France Eurocar ITAS Gilles Mas and several of the riders including Jacky Durant went to Casino Chazal Raider Mosoca Ag2r-La Mondiale Eurocar Chazal-Vetta-MBK Petit Casino Casino-AG2R Ag2r Vincent Lavenu created the Chazal team. -

Ait Annual Report on Research
Annual Report Annual 2015 AIT ANNUAL REPORT 2015 Copyright © 2016 Asian Institute of Technology. All rights reserved. POSTAL ADDRESS: STREET ADDRESS: P.O. Box 4, Klong Luang 58 Moo 9 Klong Nueng Pathumthani 12120 Km. 42 Paholyothin Highway Thailand Klong Luang, Pathumthani 12120 www.ait.asia Thailand This Annual Report was compiled and produced by Karma Rana, Izel Ann Mojado- Dante, Namita Sravat, Sanjeev Jayasinghe, Shawn Kelly, Tripti Rajbhandhari, M Zia Islam and Sarina Pradhan Thapa with the technical and editorial support of the Media and Communications Unit (MCU) and the involvement of all AIT employees. The report was designed by Nadhika Mendhaka. AIT wishes to thank the many people who rendered their assistance in preparing this report. Table of contents RESEARCH 48AWARDS AND 02THE AIT BOARD OF 28 TRUSTEES 30 AIT Projects at a Glance RECOGNITIONS 48 Faculty/Staff 52 Students 54 Alumni 32RESPONSIBILITY 03MESSAGE FROM THE CHAIRMAN OF THE AIT CENTERS BOARD OF TRUSTEES 32 School of Engineering and Technology 56AIT LIBRARY 34 School of Environment, Resources and MODERNIZATION Development 35 Internet Education and Research Laboratory (intERLab) 04MESSAGE FROM THE 36 AIT Extension PRESIDENT 38 AIT Consulting 39 AIT Center in Vietnam 60CAMPUS 41 Regional Resource Center for Asia and the REHABILITATION Pacific (RRC.AP) 42 AIT Library 43 AIT Language Center INS06TITUTIONAL 44 AIT International School (AITIS) HIGHLIGHTS 62APPENDICES 63 Financial Statement and Auditor’s Report 79 Institute Administration 80 Faculty Members 45RESOURCE 84 Collaborations and 16AcaDEMIC AFFAIRS Partnerships 16 Students at a Glance DEVELOPMENT 20 Faculty at a Glance 46 Fundraising 21 Academic Development 47 Alumni at a Glance 22 School of Engineering and Technology 24 School of Environment, Resources and Development 26 School of Management AIT ANNUAL REPORT 2015 1. -

Baird Perspectives: Cycling Industry Outlook
BAIRD PERSPECTIVES Cycling Industry Outlook How the micro-mobility and fitness revolution is impacting the bike industry. In This Report Important trends impacting the cycling industry The competition is mobilizing Winning brands will break away from the Peloton Executive Summary There is a micro-mobility and segments, especially indoor fitness revolution millennials, unfolding. On the surface, • The rise of Direct to these appear to be separate Consumer (“DTC”) revolutions, but they are oriented models with interrelated and have inherent competitive important implications for advantages, the bike industry. The way • A pronounced wealth consumers transport “multiplier themselves, the way they phenomenon” driving experience purchasing and above average growth in using a bike and the way the high-end / premium they train on a bike is segments of the outdoor undergoing a radical market, and • transformation. As a result, An increasing perception consumer perceptions and that fitness, wellness, the definition of a “bike” will access and connectivity likely never be the same. As are the new luxury. Given the rapid pace the bike industry undergoes of industry change, tectonic shifts, new and Given the rapid pace of innovative entrants will industry change, there will there will emerge and consumer undoubtedly be winners and undoubtedly be preferences and losers. While it will be winners and losers. expectations will change, difficult to determine how which will redefine the things unfold, several competitive landscape. industry actors will likely emerge big winners, Key factors impacting the including Specialized, Trek, bike industry are the Canyon and Wahoo. following: • The rise of the indoor For the winners, there will bike training and electric likely be multiple options for bike (“e-bike”) adjacent strategic categories, partnerships or exit • A growing need to opportunities. -

Maine Campus February 11 1965 Maine Campus Staff
The University of Maine DigitalCommons@UMaine Maine Campus Archives University of Maine Publications Spring 2-11-1965 Maine Campus February 11 1965 Maine Campus Staff Follow this and additional works at: https://digitalcommons.library.umaine.edu/mainecampus Repository Citation Staff, Maine Campus, "Maine Campus February 11 1965" (1965). Maine Campus Archives. 337. https://digitalcommons.library.umaine.edu/mainecampus/337 This Other is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Maine Campus Archives by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. By LINDA TOKARZ some ruling about whether to allow smoking in classrooms. Currently The projected budget for library A Serving there is no such rule. Progressive Newspaper A Growing University expenditures next year nearly Ever since the Council became doubles the current budget. ORONO, MAINE, FEBRUARY 11, 1965 aware that no rule existed, it has Head Librarian James Mac- recognized the importance of Campbell said Monday at the Fac- making one. But there has been ulty Council meeting that "the bud- a problem in semantics. With so get should be further increased by many qualifications to be con- 25 per cent each year for the next sidered—safety and health and By ELIZABETH MILLER decade" if the library facilities are housekeeping factors—the Coun- Mrs. Margaret T. Hatch is the to expand along with the univer- cil has found it impossible to University's newest assistant dean sity which they serve. of women. Mrs. Hatch started work- word a satisfactory rule. -

In Goede En Kwade Koersdagen Voor Tuur En Jef, Mijn Flandrienkes in Goede En Kwade Koersdagen Het Huwelijk Tussen Wielersport En Marketing
In goede en kwade koersdagen Voor Tuur en Jef, mijn flandrienkes In goede en kwade koersdagen Het Huwelijk tussen wielersport en marketing MARKO HEIJL Colofon auteur: Marko Heijl met dank aan: Katrien, Wim Lagae, Jos Verschueren, Rik Vanwalleghem, Stephan Vanfleteren en Nationale Loterij uitgave: Arko Sports Media Postbus 393 3430 AJ NIEUWEGEIN T. 030 707 30 00 E. [email protected] eindredactie: Janeke de Zeeuw Creatief concept: Bart Diricx – Marko Heijl foto cover: Tom Peeters DTP en realisatie: Pencilpoint - Reclamemakers & Vormgevers, Woerden fotografie en illustraties Hoewel de uitgever zijn uiterste best heeft gedaan om alle rechthebbenden van het illustratie- en fotomateriaal te achterhalen, is het mogelijk dat hij daarbij in gebreke is gebleven. In dat geval verzoeken wij u hem daarvan in kennis te stellen. Drukwerk: Drukkerij Wilco, Amersfoort ISBN 978-90-5472-157-4 NUR 489 © 2011 marko Heijl/arko sports media, nieuwegein Behoudens uitzondering door de wet gesteld mag, zonder schriftelijke toestemming van de rechthebbende(n) op het auteursrecht, c.q. de uitgever van deze uitgave door de rechthebbende(n) gemachtigd namens hem (hen) op te treden, niets uit deze uitgave worden verveelvoudigd en/of openbaar gemaakt door middel van druk, fotokopie, microfilm of anderszins, hetgeen ook van toepassing is op de gehele of gedeeltelijke bewerking. De uitgever is met uitsluiting van ieder ander gerechtigd de door derden verschuldigde vergoedingen voor kopiëren, als bedoeld in art. 17 lid 2. Auteurswet 1912 en in het KB van 20 juni -

The Effect of Acquisition Announcements on Stock Returns of Acquiring Firms
A Work Project, presented as part of the requirements for the Award of an International Master in Finance from the NOVA – School of Business and Economics and a Professional Master in Finance from the Fundação Getulio Vargas – São Paulo School of Economics THE EFFECT OF ACQUISITION ANNOUNCEMENTS ON STOCK RETURNS OF ACQUIRING FIRMS: A SHORT- AND LONG-TERM STUDY FOR DEVELOPED AND EMERGING COUNTRIES FOCUSING ON DOMESTIC AND CROSS-BORDER ACQUISITIONS AND THE IMPACT OF INSTITUTIONAL ENVIRONMENT Florian Otto NOVA SBE Student numbers: 3363 & 29365 FGV-EESP Student number: 338939 A Project carried out on the Double Degree EESP-FGV, under supervision of: Professor Rafael Zambrana (NOVA SBE, Lisbon, Portugal) Professor Joelson Sampaio (EESP-FGV, São Paulo, Brazil) Date: 10th September 2017 Abstract This paper uses an empirical event-study approach to investigate the effect of acquisition announcements on the share price of bidding firms. By using a globalised set of acquirers from 14 different markets, this work aims to develop an understanding of the impact of acquisition announcements on developed- and emerging-market acquirers. The sample contains 624 acquisition announcements with target firms located throughout the world between 1997 and 2015. The results contradict the conventional wisdom that developed-market acquirers generally experience losses. Both emerging-market and developed-market bidders gain significantly in the short term. Moreover, for developed-market acquirers, the announcement of cross-border acquisitions (CBAs) yields higher abnormal returns than the announcement of domestic acquisitions. On the other hand, emerging-market bidders gain from announcing domestic acquisitions, and lose substantially when publishing news about CBAs. -

Vainqueurs D'étapes Du Tour D'italie (Le Giro)
Vainqueurs d'étapes du Tour d'Italie (Le Giro) Vainqueurs d'étapes du Tour d'Italie (Le Giro) depuis 1909. Etapes du 08/05 au 30/05/2021 1 Torino - Torino, 8.6 km C.M.Individuel 2 Stupinigi - Novara, 179 km 3 Biella - Canale, 190 km 4 Piacenza - Sestola, 187 km 5 Modena - Cattolica, 177 km 6 Grotte di Frasassi - Ascoli Piceno, 160 km 7 Notaresco - Termoli, 181 km 8 Foggia - Guardia Sanframondi, 170 km 9 Castel di Sangro - Campo Felice, 158 km 10 L'Aquila - Foligno, 139 km 11 Perugia - Montalcino, 162 km 12 Siena - Bagno di Romagna, 212 km 13 Ravenna - Verona, 198 km 14 Cittadella - Monte Zoncolan, 205 km 15 Grado - Gorizia, 147 km 16 Sacile - Cortina d'Ampezzo, 153 km 17 Canazei - Sega di Ala, 193 km 18 Rovereto - Stradella, 231 km 19 Abbiategrasso - Alpe di Mera, 176 km 20 Verbania - Valle Spluga-Alpe Motta, 164 km 21 Milano - Milano, 30.3 km C.M.Individuel 1ère étape. 1er Filippo Ganna (Ita) C.M.Individuel 2ème étape. 1er Tim Merlier (Bel) 3ème étape. 1er Taco Van Der Hoorn (Hol) 4ème étape. 1er Joseph Lloyd Dombrowski (Usa) 5ème étape. 1er Caleb Ewan (Aus) 6ème étape. 1er Gino Mäder (Sui) 7ème étape. 1er Caleb Ewan (Aus) 8ème étape. 1er Victor Lafay (Fra) 9ème étape. 1er Egan Arley Bernal Gomez (Col) 10ème étape. 1er Peter Sagan (Svq) 11ème étape. 1er Mauro Schmid (Sui) 12ème étape. 1er Andrea Vendrame (Ita) 13ème étape. 1er Giacomo Nizzolo (Ita) 14ème étape. 1er Lorenzo Fortunato (Ita) 15ème étape. 1er Victor Campenaerts (Bel) 16ème étape. -

The Lithium-Ion Battery Value Chain
THE LITHIUM-ION BATTERY VALUE CHAIN New Economy Opportunities for Australia Acknowledgment Austrade would like to express our appreciation to Future Smart Strategies, especially Howard Buckley, for his professional guidance, advice and assistance, with earlier versions of this report. We would also like to thank Adrian Griffin at Lithium Australia for his insights and constructive suggestions. And we would like to acknowledge the insights provided by Prabhav Sharma at McKinsey & Company. More broadly, we would like to thank the following companies and organisations for providing data and information that assisted our research: › Association of Mining and Exploration Australia (AMEC); › Geoscience Australia; › Albemarle; and › TianQi Australia. Disclaimer Copyright © Commonwealth of Australia 2018 This report has been prepared by the Commonwealth of Australia represented by the Australian Trade and Investment Commission (Austrade). The report is a general overview and is not intended to The material in this document is licensed under a Creative Commons provide exhaustive coverage of the topic. The information is made Attribution – 4.0 International licence, with the exception of: available on the understanding that the Commonwealth of Australia is • the Australian Trade and Investment Commission’s logo not providing professional advice. • any third party material While care has been taken to ensure the information in this report • any material protected by a trade mark is accurate, the Commonwealth does not accept any liability for any • any images and photographs. loss arising from reliance on the information, or from any error or More information on this CC BY licence is set out at the creative omission, in the report. -

Alex Graham Simon-Kucher and Partners
Financial services forum Pricing in a digital world 4 April 2017 Alex Graham, Director London office 1 Plough Place London EC4A 1DE, UK Tel. +44 20 7832 6700 [email protected] www.simon-kucher.com Simon-Kucher in numbers 980 97 £197m employees worldwide partners annual turnover 2016 17% 3,200 #1 growth rate* projects in the last ranked consultancy three years in Marketing, Pricing, Sales and CRM** * 10 year CAGR; ** manager magazin survey of top managers in Germany, August 2007 and 2011/IMB; brand eins Thema/Statista survey of 1,426 partners and project leaders from consulting firms and 1,300 C-level managers from German companies, May 2014 Pricing in a digital world conference: Retail banking 2 The world's leading pricing adviser "world leader in giving advice to companies on "…the world's how to price their leading pricing products" consultancy…" Peter Drucker "... in pricing you "pricing strategy offer something specialists" nobody else does." The influence of Professor Philip Kotler Simon-Kucher on the prices we pay for just “No one knows more about about everything is as pricing than SKP." little recognised as it is staggering Source: BusinessWeek, January 18, 2004; The Wall Street Journal, September 22, 2003; letter to Hermann Simon, Simon-Kucher's founder and chairman, June 3, 2003; "Business Consulting", Gilbert & Czerniawska, 2005, The Economist Books; William Poundstone 2010 Pricing in a digital world conference: Retail banking 3 Simon-Kucher works with industry leaders from all sectors Automotive Consumer & Retail -

MARITIME REPORTER and Cyber Security ENGINEERING NEWS Are Your Vessels Secure? Are You Sure?
COV1 MR July 2012 V2:COVER PAGE.qxd 7/9/2012 12:09 PM Page 1 The World’s Largest Circulation Marine Industry Publication • The Information Authority for the Global Marine Industry since 1939 JULY 2012 MARITIME REPORTER AND Cyber Security ENGINEERING NEWS Are your vessels secure? Are you sure? MARINELINK.COM Arctic Bound (with Vigor) Vigor Industrial leads a surge in the Pacific NW Government Update USCG Goes Big in Arctic Legal Beat U.S. & the “Law of the Sea” Propulsion Avoid Catastrophic Failures Security Maritime Cyber Security Slow Steaming Benefits & Problems Spill Response Ice & Oil do not Mix COV2,C3&C4 MR July 12:COV2,C3&C4 MR May.09.qxd 7/3/2012 10:37 AM Page 1 MR#7 (1-9):MR Template 7/3/2012 11:03 AM Page 1 MR#7 (1-9):MR Template 7/9/2012 3:46 PM Page 2 contents Covered Up Driven by new rules and regulation, marine coating companies have been driven to develop new prod- ucts that are increasingly environmentally benign and at the same time more rugged and cost-efficient for the shipowner. Five leading marine coating executives weigh in on the burning questions of the day, starting on page 30. (Photo: Hempel) ON THE COVER 20 As ships, boats and rigs in- creasingly become an ex- tension of the land-based 12 ARCTIC SHIELD 2012 24 ARCTIC BOUND (WITH VIGOR) office — using high speed, While offshore oil and gas garners much of the Arctic attention, the Vigor Industrial has led the charge to reinvigorate maritime business high volume comms and so- USCG is today assembling its largest ever effort up north.