February 2, 2021 Gemtier Medical (Shanghai)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World Bank for OFFICIAL USE ONLY
Document of The World Bank FOR OFFICIAL USE ONLY Public Disclosure Authorized Report No: 49565-CN PROJECT APPRAISAL DOCUMENT ON A Public Disclosure Authorized PROPOSED GRANT FROM THE GLOBAL ENVIR0NMEN.T FACILITY TRUST FUND IN THE AMOUNT OF US$4.788 MILLION TO THE PEOPLE’S REPUBLIC OF CHINA FOR A Public Disclosure Authorized SHANGHAI AGRICULTURAL AND NON-POINT POLLUTION REDUCTION PROJECT May 18,2010 China and Mongolia Sustainable Development Unit Sustainable Development Department East Asia and Pacific Region This document has a restricted distribution and may be used by recipients only in the Public Disclosure Authorized performance of their official duties. Its contents may not otherwise be disclosed without World Bank authorization. CURRENCY EQUIVALENTS (Exchange Rate Effective September 29, 2009) Currency Unit = Renminbi Yuan (RMB) RMB6.830 = US$1 US$0.146 = RMB 1 FISCAL YEAR January 1 - December31 ABBREVIATIONS AND ACRONYMS APL Adaptable Program Loan AMP Abbreviated Resettlement Action Plan BOD Biological Oxygen Demand CAS Country Assistance Strategy CDM Clean Development Mechanism CEA Consolidated Project- Wide Environmental Assessment CEMP Consolidated Project- Wide Environmental Management Plan CNAO China National Audit Office COD Chemical Oxygen Demand CSTR Completely Stirred Tank Reactor DA Designated Account EA Environmental Assessment ECNU East China Normal University EIRR Economic Internal Rate of Return EMP Environmental Management Plan ER Emission Reduction FA0 Food and Agricultural Organization FM Financial Management FMM -

Press Release
PRESS RELEASE Merlin Entertainments announces LEGOLAND Shanghai Resort is anticipated to open in 2024 Construction of one of the largest LEGOLAND parks in the world expected to begin in 2021 6 November 2020: Merlin Entertainments (“Merlin” or “the Company”), a global leader in location based entertainment with brands including LEGOLAND®, Madame Tussauds and the Dungeons, today announces that it has entered into a formal co-operation agreement with the Shanghai Jinshan District Government, CMC Inc. and KIRKBI to develop a LEGOLAND® Resort in the Jinshan District of Shanghai, China. This follows the signing of a framework agreement in November 2019, announced as part of the China International Import Expo. Under the terms of the agreement, all parties will form a joint venture company and contribute funding to the construction and development of LEGOLAND® Shanghai. The total project investment is expected to be approximately $550 million. Construction of the project is planned to start next year, and the Resort is expected to open in 2024. LEGOLAND® Shanghai will be one of the largest LEGOLAND® Resorts in the world and will incorporate a 250-room fully themed hotel on opening. World-leading creative, design and construction teams will work together to create an immersive theme park, drawing inspiration from famous scenic spots in Shanghai, Jinshan District and the town of Fengjing. It will be located in the Jinshan District in south west Shanghai with a two- hour catchment of 55 million people. The region comprising Shanghai, Jiangsu, Zhejiang and Anhui has an estimated population of 220 million. China is a focus of significant development and investment by Merlin. -

How Shanghai Does It
How Shanghai Does ItHow DIRECTIONS IN DEVELOPMENT Human Development Liang, Kidwai, and Zhang Liang, How Shanghai Does It Insights and Lessons from the Highest-Ranking Education System in the World Xiaoyan Liang, Huma Kidwai, and Minxuan Zhang How Shanghai Does It DIRECTIONS IN DEVELOPMENT Human Development How Shanghai Does It Insights and Lessons from the Highest-Ranking Education System in the World Xiaoyan Liang, Huma Kidwai, and Minxuan Zhang © 2016 International Bank for Reconstruction and Development / The World Bank 1818 H Street NW, Washington, DC 20433 Telephone: 202-473-1000; Internet: www.worldbank.org Some rights reserved 1 2 3 4 19 18 17 16 This work is a product of the staff of The World Bank with external contributions. The findings, interpreta- tions, and conclusions expressed in this work do not necessarily reflect the views of The World Bank, its Board of Executive Directors, or the governments they represent. The World Bank does not guarantee the accuracy of the data included in this work. The boundaries, colors, denominations, and other information shown on any map in this work do not imply any judgment on the part of The World Bank concerning the legal status of any territory or the endorsement or acceptance of such boundaries. Nothing herein shall constitute or be considered to be a limitation upon or waiver of the privileges and immunities of The World Bank, all of which are specifically reserved. Rights and Permissions This work is available under the Creative Commons Attribution 3.0 IGO license (CC BY 3.0 IGO) http:// creativecommons.org/licenses/by/3.0/igo. -
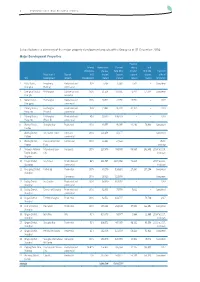
Major Development Properties
1 SHANGHAI INDUSTRIAL HOLDINGS LIMITED Set out below is a summary of the major property development projects of the Group as at 31 December 2016: Major Development Properties Pre-sold Interest Approximate Planned during Total attributable site area total GFA the year GFA sold Expected Projects of SI Type of to SI (square (square (square (square date of City Development property Development meters) meters) meters) meters) completion 1 Kaifu District, Fengsheng Residential and 90% 5,468 70,566 7,542 – Completed Changsha Building commercial 2 Chenghua District, Hi-Shanghai Commercial and 100% 61,506 254,885 75,441 151,644 Completed Chengdu residential 3 Beibei District, Hi-Shanghai Residential and 100% 30,845 74,935 20,092 – 2019 Chongqing commercial 4 Yuhang District, Hi-Shanghai Residential and 85% 74,864 230,484 81,104 – 2019 Hangzhou (Phase I) commercial 5 Yuhang District, Hi-Shanghai Residential and 85% 59,640 198,203 – – 2019 Hangzhou (Phase II) commercial 6 Wuxing District, Shanghai Bay Residential 100% 85,555 96,085 42,236 76,966 Completed Huzhou 7 Wuxing District, SIIC Garden Hotel Hotel and 100% 116,458 47,177 – – Completed Huzhou commercial 8 Wuxing District, Hurun Commercial Commercial 100% 13,661 27,322 – – Under Huzhou Plaza planning 9 Shilaoren National International Beer Composite 100% 227,675 783,500 58,387 262,459 2014 to 2018, Tourist Resort, City in phases Qingdao 10 Fengze District, Sea Palace Residential and 49% 381,795 1,670,032 71,225 – 2017 to 2021, Quanzhou commercial in phases 11 Changning District, United 88 Residential -
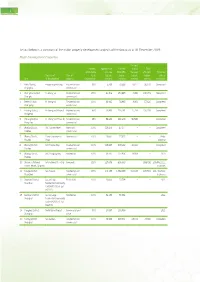
Set out Below Is a Summary of the Major Property Development Projects of the Group As at 31 December 2019: Major Development
1 Set out below is a summary of the major property development projects of the Group as at 31 December 2019: Major Development Properties Pre-sold Interest Approximate Planned during Total attributable site area total GFA the year GFA sold Expected Projects of Type of to SI (square (square (square (square date of City SI Development property Development meters) meters) meters) meters) completion 1 Kaifu District, Fengsheng Building Residential and 90% 5,468 70,566 6,627 30,870 Completed Changsha commercial 2 Chenghua District, Hi-Shanghai Residential and 100% 61,506 254,885 4,996 190,153 Completed Chengdu commercial 3 Beibei District, Hi-Shanghai Residential and 100% 30,845 74,935 3,301 57,626 Completed Chongqing commercial 4 Yuhang District, Hi-Shanghai (Phase I) Residential and 85% 74,864 230,484 27,758 150,289 Completed Hangzhou commercial 5 Yuhang District, Hi-Shanghai (Phase II) Residential and 85% 59,640 198,203 56,539 – Completed Hangzhou commercial 6 Wuxing District, SIIC Garden Hotel Hotel and 100% 116,458 47,177 – – Completed Huzhou commercial 7 Wuxing District, Hurun Commercial Commercial 100% 13,661 27,322 – – Under Huzhou Plaza planning 8 Wuxing District, SIIC Tianlan Bay Residential and 100% 115,647 193,292 26,042 – Completed Huzhou commercial 9 Wuxing District, SIIC Yungjing Bay Residential 100% 68,471 207,906 28,953 – 2020 Huzhou 10 Shilaoren National International Beer City Composite 100% 227,675 806,339 – 333,798 2014 to 2022, Tourist Resort, Qingdao in phases 11 Fengze District, Sea Palace Residential and 100% 170,133 -

(2021-04-29)Summary of Quarterly Solvency Report (China Pacific Anxin Agricultural Insurance Company Limited)
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. 中國太平洋保險(集團)股份有限公司 CHINA PACIFIC INSURANCE (GROUP) CO., LTD. (A joint stock company incorporated in the People’s Republic of China with limited liability) (Stock Code: 02601) Overseas Regulatory Announcement This overseas regulatory announcement is made pursuant to Rule 13.09 and Rule 13.10B of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited (the “Listing Rules”) and the Inside Information Provisions (as defined in the Listing Rules) under Part XIVA of the Securities and Futures Ordinance (Chapter 571 of the Laws of Hong Kong). The announcement is attached hereof for information purpose only. By Order of the Board China Pacific Insurance (Group) Co., Ltd. KONG Qingwei Chairman Hong Kong, 29 April 2021 As at the date of this announcement, the Executive Directors of the Company are Mr. KONG Qingwei and Mr. FU Fan; the Non-executive Directors are Mr. HUANG Dinan, Mr. WANG Tayu, Mr. WU Junhao, Mr. CHEN Ran, Mr. ZHOU Donghui, Ms. LIANG Hong, Ms. LU Qiaoling and Mr. John Robert DACEY; and the Independent Non-executive Directors are Ms. LIU Xiaodan, Mr. CHEN Jizhong, Ms. LAM Tyng Yih, Elizabeth, Mr. WOO Ka Biu, Jackson and Mr. JIANG Xuping. Summary of Quarterly Solvency Report China Pacific Anxin Agricultural Insurance Company Limited 1st Quarter of 2021 Contents I. -

Activities by Region China
Activities by Region China China ・Mazda officially entered the Chinese market in 2001 and established a local 3 affiliate company in 2005 to implement a unified brand strategy over two sales 2 channels, FAW Mazda and Changan Mazda. ・The Japan-produced all-new Mazda6 went on sale in China in late June 2013, and 4 1 production of the CX-5 started at the Nanjing plant in July. Mazda Atenza (Imported from Japan) CX-5 (Produced in China) Regional Offices (As of December 31, 2012) Distributors (As of December 31, 2012) Country/ Number of Investment Country/ Number of Company name Location Established Primary business Company name Location Established Investment ratio Region employees ratio region employees Mazda Motor (China) Co., Ltd. Pudong New District, January Overall management FAW Car 56% 1 Mazda 100% (MCO) Shanghai 2005 of business in China FAW Mazda Motor Sales Co., Ltd. (FMSC) Changchun, Jilin Province March 2005 311 Mazda 40% Mazda Motor (China) Co., Ltd. Chaoyang District, November FAW Group 4% 2 Branch Office of MCO - China Beijing Branch( MCO-Beijing) Beijing 2007 Changan Mazda Automobile Corporation, LTD. Sales department of Nanjing, Jiangsu Province April 2007 266 China 101 Branch Office of MCO/ Workshops, Sales branch (CAM) CFMA Mazda Motor (China) Co., Ltd. market research and technology Jiading District, August 1 China Engineering Support Center studies for the Chinese market, - Shanghai 2005 (MCO-CESC) as well as technical support in the region Mazda Vehicle Production (As of December 31, 2012) (Units) CY2008 CY2009 CY2010 CY2011 CY2012 FAW Car Co., Ltd. 65,670 101,844 139,635 128,325 102,372 China Changan Ford Mazda Automobile Co., Ltd. -

Development of High-Speed Rail in the People's Republic of China
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Haixiao, Pan; Ya, Gao Working Paper Development of high-speed rail in the People's Republic of China ADBI Working Paper Series, No. 959 Provided in Cooperation with: Asian Development Bank Institute (ADBI), Tokyo Suggested Citation: Haixiao, Pan; Ya, Gao (2019) : Development of high-speed rail in the People's Republic of China, ADBI Working Paper Series, No. 959, Asian Development Bank Institute (ADBI), Tokyo This Version is available at: http://hdl.handle.net/10419/222726 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. may exercise further usage rights as specified in the indicated licence. https://creativecommons.org/licenses/by-nc-nd/3.0/igo/ www.econstor.eu ADBI Working Paper Series DEVELOPMENT OF HIGH-SPEED RAIL IN THE PEOPLE’S REPUBLIC OF CHINA Pan Haixiao and Gao Ya No. -

Effectiveness of Live Poultry Market Interventions on Human Infection with Avian Influenza A(H7N9) Virus, China Wei Wang,1 Jean Artois,1 Xiling Wang, Adam J
RESEARCH Effectiveness of Live Poultry Market Interventions on Human Infection with Avian Influenza A(H7N9) Virus, China Wei Wang,1 Jean Artois,1 Xiling Wang, Adam J. Kucharski, Yao Pei, Xin Tong, Victor Virlogeux, Peng Wu, Benjamin J. Cowling, Marius Gilbert,2 Hongjie Yu2 September 2017) as of March 2, 2018 (2). Compared Various interventions for live poultry markets (LPMs) have with the previous 4 epidemic waves, the 2016–17 fifth emerged to control outbreaks of avian influenza A(H7N9) virus in mainland China since March 2013. We assessed wave raised global concerns because of several char- the effectiveness of various LPM interventions in reduc- acteristics. First, a surge in laboratory-confirmed cas- ing transmission of H7N9 virus across 5 annual waves es of H7N9 virus infection was observed in wave 5, during 2013–2018, especially in the final wave. With along with some clusters of limited human-to-human the exception of waves 1 and 4, various LPM interven- transmission (3,4). Second, a highly pathogenic avi- tions reduced daily incidence rates significantly across an influenza H7N9 virus infection was confirmed in waves. Four LPM interventions led to a mean reduction Guangdong Province and has caused further human of 34%–98% in the daily number of infections in wave 5. infections in 3 provinces (5,6). The genetic divergence Of these, permanent closure provided the most effective of H7N9 virus, its geographic spread (7), and a much reduction in human infection with H7N9 virus, followed longer epidemic duration raised concerns about an by long-period, short-period, and recursive closures in enhanced potential pandemic threat in 2016–17. -

Supplementary Material
1 Supplementary Material 2 3 4 Energy and Material Flows of Megacities 5 6 Chris Kennedy*1 7 Iain Stewart1 8 Angelo Facchini2 9 Igor Cersosimo2 10 Renata Mele2 11 Bin Chen3 12 Mariko Uda1 13 Arun Kansal4 14 Anthony Chiu5 15 Kwi-gon Kim6 16 Carolina Dubeux7 17 Emilio Lebre La Rovere7 18 Bruno Cunha 7 19 Stephanie Pincetl8 20 James Keirstead9 21 Sabine Barles10 22 Semerdanta Pusaka11 23 Juniati Gunawan11 24 Michael Adegbile12 25 Mehrdad Nazariha13 26 Shamsul Hoque14 27 Peter Marcotullio15 28 Florencia Gonzalezo16 29 Tarek Genena17 30 Nadine Ibrahim1 31 Rizwan Farooqui18 32 Gemma Cervantes19 33 Ahmet Duran Sahin20 34 35 * corresponding author 36 37 1: Department of Civil Engineering, University of Toronto, 35 St. George Street, 38 Toronto, Ontario. CANADA. M4J 3K1. Tel: +1 416 978 5978 39 2: Enel Foundation, 00198, Viale Regina Margherita n. 137, Rome, Italy 40 3: School of Environment, Beijing Normal University, China, 北京市海淀区新街口外 41 大街19号 邮政编码: 100875 42 4: Department of Energy and Environment, TERI University, 10 Institutional Area, 43 Vasant Kunj, New Delhi, DL 110070, India 44 5: Department of Industrial Engineering, De La Salle University, 2401 Taft Ave, Malate, 45 Manila, 1004 Metro Manila, Philippines 1 46 6: Department of Landscape and Ecological Planning, Seoul National University, 1 47 Gwanak-ro, Gwanak-gu, Seoul, South Korea 48 7: COPPE, Federal University of Rio de Janeiro, Av. Pedro Calmon, 550 - Cidade 49 Universitária, Rio de Janeiro - RJ, 21941-901, Brazil 50 8: UCLA Institute of the Environment and Sustainability, La Kretz Hall, Suite 300, Los 51 Angeles, CA 90095-1496. -

Case Study Shanghai, Jiading Riverfront Masterplan Competition
Case study Shanghai, Jiading Riverfront Masterplan competition A Design vision that connects the three cities via new boulevards and canals. A Year The opportunity Our contribution 2013 Beijing-based HYHW Architects were Using in-depth case study analyses invited to join a design competition for of large urban conurbations such as Professional team the creation of a regional masterplan Barcelona and London, a set of key HYHW Architects in the Jiading district to the north- design principles was developed and west of Shanghai. The large site used to assess the existing situation Our role is located between Jiading City, and to develop the emerging design Strategic urban design advisor Nanxiang City and Anting City. The with HYHW in terms of spatial layout, connections between the three land use strategy and transport Key features cities are currently constrained by a planning. The design vision connects Case study analysis vehicle traffic-oriented infrastructure, the three town centres (yellow circles) Strategic design advice disconnected street network and through boulevards (red lines), the a poor quality public realm. Space central canal (blue line) and north- Syntax was appointed to undertake south links (green lines). analysis to provide the team with an understanding of the opportunities and The outcome constraints of the site and to assist in Space Syntax’s recommendations developing strategic design proposals. were communicated to the design team throughout the competition process and are therefore embedded in the final proposals. [email protected] www.spacesyntax.com C Spatial layout analysis of the proposed masterplan showing strong urban connections in red. D Vision concept. -

Getting up from a Chair, Getting Up, Dressing and Eating
Technology and Health Care 28 (2020) S263–S271 S263 DOI 10.3233/THC-209027 IOS Press Analysis of behavioral disability and construction of a disability scale for the elderly in Shanghai Zeguo Shaoa;b, Yuhong Xianga;∗, Mina Yanb and Wei Chenb;c;∗ aShanghai University of Medicine and Health Sciences, Shanghai, China bCenter for Intelligent Medical Electronics, School of Information Science and Engineering, Fudan University, Shanghai, China cShanghai Key Laboratory of Medical Imaging Computing and Computer Assisted Intervention, Shanghai, China Abstract. BACKGROUND: With the gradual aging of China’s population development, the number of disabled elderly has increased significantly. OBJECTIVE: In order to better solve the problem of life care for these elderly people, it is necessary to conduct in-depth and detailed research on the specific conditions of disabled elderly people, in order to differentiate different conditions for care and set appropriate insurance provisions. METHODS: Based on the detailed analysis of the basic behavioral ability of the elderly, and referring to the International Disability standards, this paper refines the three basic living ability indicators of physiological behavior, cognitive behavior and interpersonal behavior, and integrates the cultural elements of assimilation, continuity, fusion and cohesion with Chinese characteristics. A more systematic and perfect five-level disability scale which conforms to the national conditions of China is designed. RESULTS: The disability of the elderly in Shanghai was investigated with the newly constructed scale, and a detailed analysis and five-level division were made. CONCLUSION: Experiments show that the results of this study can more effectively establish the disability level of the elderly in China.