An Acute Gastroenteritis Outbreak Associated with Person-To-Person
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World Bank for OFFICIAL USE ONLY
Document of The World Bank FOR OFFICIAL USE ONLY Public Disclosure Authorized Report No: 49565-CN PROJECT APPRAISAL DOCUMENT ON A Public Disclosure Authorized PROPOSED GRANT FROM THE GLOBAL ENVIR0NMEN.T FACILITY TRUST FUND IN THE AMOUNT OF US$4.788 MILLION TO THE PEOPLE’S REPUBLIC OF CHINA FOR A Public Disclosure Authorized SHANGHAI AGRICULTURAL AND NON-POINT POLLUTION REDUCTION PROJECT May 18,2010 China and Mongolia Sustainable Development Unit Sustainable Development Department East Asia and Pacific Region This document has a restricted distribution and may be used by recipients only in the Public Disclosure Authorized performance of their official duties. Its contents may not otherwise be disclosed without World Bank authorization. CURRENCY EQUIVALENTS (Exchange Rate Effective September 29, 2009) Currency Unit = Renminbi Yuan (RMB) RMB6.830 = US$1 US$0.146 = RMB 1 FISCAL YEAR January 1 - December31 ABBREVIATIONS AND ACRONYMS APL Adaptable Program Loan AMP Abbreviated Resettlement Action Plan BOD Biological Oxygen Demand CAS Country Assistance Strategy CDM Clean Development Mechanism CEA Consolidated Project- Wide Environmental Assessment CEMP Consolidated Project- Wide Environmental Management Plan CNAO China National Audit Office COD Chemical Oxygen Demand CSTR Completely Stirred Tank Reactor DA Designated Account EA Environmental Assessment ECNU East China Normal University EIRR Economic Internal Rate of Return EMP Environmental Management Plan ER Emission Reduction FA0 Food and Agricultural Organization FM Financial Management FMM -

Press Release
PRESS RELEASE Merlin Entertainments announces LEGOLAND Shanghai Resort is anticipated to open in 2024 Construction of one of the largest LEGOLAND parks in the world expected to begin in 2021 6 November 2020: Merlin Entertainments (“Merlin” or “the Company”), a global leader in location based entertainment with brands including LEGOLAND®, Madame Tussauds and the Dungeons, today announces that it has entered into a formal co-operation agreement with the Shanghai Jinshan District Government, CMC Inc. and KIRKBI to develop a LEGOLAND® Resort in the Jinshan District of Shanghai, China. This follows the signing of a framework agreement in November 2019, announced as part of the China International Import Expo. Under the terms of the agreement, all parties will form a joint venture company and contribute funding to the construction and development of LEGOLAND® Shanghai. The total project investment is expected to be approximately $550 million. Construction of the project is planned to start next year, and the Resort is expected to open in 2024. LEGOLAND® Shanghai will be one of the largest LEGOLAND® Resorts in the world and will incorporate a 250-room fully themed hotel on opening. World-leading creative, design and construction teams will work together to create an immersive theme park, drawing inspiration from famous scenic spots in Shanghai, Jinshan District and the town of Fengjing. It will be located in the Jinshan District in south west Shanghai with a two- hour catchment of 55 million people. The region comprising Shanghai, Jiangsu, Zhejiang and Anhui has an estimated population of 220 million. China is a focus of significant development and investment by Merlin. -
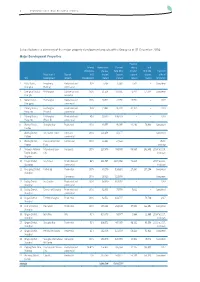
Major Development Properties
1 SHANGHAI INDUSTRIAL HOLDINGS LIMITED Set out below is a summary of the major property development projects of the Group as at 31 December 2016: Major Development Properties Pre-sold Interest Approximate Planned during Total attributable site area total GFA the year GFA sold Expected Projects of SI Type of to SI (square (square (square (square date of City Development property Development meters) meters) meters) meters) completion 1 Kaifu District, Fengsheng Residential and 90% 5,468 70,566 7,542 – Completed Changsha Building commercial 2 Chenghua District, Hi-Shanghai Commercial and 100% 61,506 254,885 75,441 151,644 Completed Chengdu residential 3 Beibei District, Hi-Shanghai Residential and 100% 30,845 74,935 20,092 – 2019 Chongqing commercial 4 Yuhang District, Hi-Shanghai Residential and 85% 74,864 230,484 81,104 – 2019 Hangzhou (Phase I) commercial 5 Yuhang District, Hi-Shanghai Residential and 85% 59,640 198,203 – – 2019 Hangzhou (Phase II) commercial 6 Wuxing District, Shanghai Bay Residential 100% 85,555 96,085 42,236 76,966 Completed Huzhou 7 Wuxing District, SIIC Garden Hotel Hotel and 100% 116,458 47,177 – – Completed Huzhou commercial 8 Wuxing District, Hurun Commercial Commercial 100% 13,661 27,322 – – Under Huzhou Plaza planning 9 Shilaoren National International Beer Composite 100% 227,675 783,500 58,387 262,459 2014 to 2018, Tourist Resort, City in phases Qingdao 10 Fengze District, Sea Palace Residential and 49% 381,795 1,670,032 71,225 – 2017 to 2021, Quanzhou commercial in phases 11 Changning District, United 88 Residential -
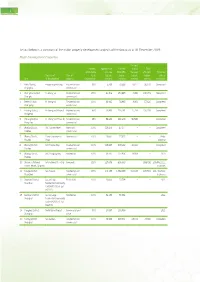
Set out Below Is a Summary of the Major Property Development Projects of the Group As at 31 December 2019: Major Development
1 Set out below is a summary of the major property development projects of the Group as at 31 December 2019: Major Development Properties Pre-sold Interest Approximate Planned during Total attributable site area total GFA the year GFA sold Expected Projects of Type of to SI (square (square (square (square date of City SI Development property Development meters) meters) meters) meters) completion 1 Kaifu District, Fengsheng Building Residential and 90% 5,468 70,566 6,627 30,870 Completed Changsha commercial 2 Chenghua District, Hi-Shanghai Residential and 100% 61,506 254,885 4,996 190,153 Completed Chengdu commercial 3 Beibei District, Hi-Shanghai Residential and 100% 30,845 74,935 3,301 57,626 Completed Chongqing commercial 4 Yuhang District, Hi-Shanghai (Phase I) Residential and 85% 74,864 230,484 27,758 150,289 Completed Hangzhou commercial 5 Yuhang District, Hi-Shanghai (Phase II) Residential and 85% 59,640 198,203 56,539 – Completed Hangzhou commercial 6 Wuxing District, SIIC Garden Hotel Hotel and 100% 116,458 47,177 – – Completed Huzhou commercial 7 Wuxing District, Hurun Commercial Commercial 100% 13,661 27,322 – – Under Huzhou Plaza planning 8 Wuxing District, SIIC Tianlan Bay Residential and 100% 115,647 193,292 26,042 – Completed Huzhou commercial 9 Wuxing District, SIIC Yungjing Bay Residential 100% 68,471 207,906 28,953 – 2020 Huzhou 10 Shilaoren National International Beer City Composite 100% 227,675 806,339 – 333,798 2014 to 2022, Tourist Resort, Qingdao in phases 11 Fengze District, Sea Palace Residential and 100% 170,133 -

Development of High-Speed Rail in the People's Republic of China
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Haixiao, Pan; Ya, Gao Working Paper Development of high-speed rail in the People's Republic of China ADBI Working Paper Series, No. 959 Provided in Cooperation with: Asian Development Bank Institute (ADBI), Tokyo Suggested Citation: Haixiao, Pan; Ya, Gao (2019) : Development of high-speed rail in the People's Republic of China, ADBI Working Paper Series, No. 959, Asian Development Bank Institute (ADBI), Tokyo This Version is available at: http://hdl.handle.net/10419/222726 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. may exercise further usage rights as specified in the indicated licence. https://creativecommons.org/licenses/by-nc-nd/3.0/igo/ www.econstor.eu ADBI Working Paper Series DEVELOPMENT OF HIGH-SPEED RAIL IN THE PEOPLE’S REPUBLIC OF CHINA Pan Haixiao and Gao Ya No. -

Effectiveness of Live Poultry Market Interventions on Human Infection with Avian Influenza A(H7N9) Virus, China Wei Wang,1 Jean Artois,1 Xiling Wang, Adam J
RESEARCH Effectiveness of Live Poultry Market Interventions on Human Infection with Avian Influenza A(H7N9) Virus, China Wei Wang,1 Jean Artois,1 Xiling Wang, Adam J. Kucharski, Yao Pei, Xin Tong, Victor Virlogeux, Peng Wu, Benjamin J. Cowling, Marius Gilbert,2 Hongjie Yu2 September 2017) as of March 2, 2018 (2). Compared Various interventions for live poultry markets (LPMs) have with the previous 4 epidemic waves, the 2016–17 fifth emerged to control outbreaks of avian influenza A(H7N9) virus in mainland China since March 2013. We assessed wave raised global concerns because of several char- the effectiveness of various LPM interventions in reduc- acteristics. First, a surge in laboratory-confirmed cas- ing transmission of H7N9 virus across 5 annual waves es of H7N9 virus infection was observed in wave 5, during 2013–2018, especially in the final wave. With along with some clusters of limited human-to-human the exception of waves 1 and 4, various LPM interven- transmission (3,4). Second, a highly pathogenic avi- tions reduced daily incidence rates significantly across an influenza H7N9 virus infection was confirmed in waves. Four LPM interventions led to a mean reduction Guangdong Province and has caused further human of 34%–98% in the daily number of infections in wave 5. infections in 3 provinces (5,6). The genetic divergence Of these, permanent closure provided the most effective of H7N9 virus, its geographic spread (7), and a much reduction in human infection with H7N9 virus, followed longer epidemic duration raised concerns about an by long-period, short-period, and recursive closures in enhanced potential pandemic threat in 2016–17. -

Supplementary Material
1 Supplementary Material 2 3 4 Energy and Material Flows of Megacities 5 6 Chris Kennedy*1 7 Iain Stewart1 8 Angelo Facchini2 9 Igor Cersosimo2 10 Renata Mele2 11 Bin Chen3 12 Mariko Uda1 13 Arun Kansal4 14 Anthony Chiu5 15 Kwi-gon Kim6 16 Carolina Dubeux7 17 Emilio Lebre La Rovere7 18 Bruno Cunha 7 19 Stephanie Pincetl8 20 James Keirstead9 21 Sabine Barles10 22 Semerdanta Pusaka11 23 Juniati Gunawan11 24 Michael Adegbile12 25 Mehrdad Nazariha13 26 Shamsul Hoque14 27 Peter Marcotullio15 28 Florencia Gonzalezo16 29 Tarek Genena17 30 Nadine Ibrahim1 31 Rizwan Farooqui18 32 Gemma Cervantes19 33 Ahmet Duran Sahin20 34 35 * corresponding author 36 37 1: Department of Civil Engineering, University of Toronto, 35 St. George Street, 38 Toronto, Ontario. CANADA. M4J 3K1. Tel: +1 416 978 5978 39 2: Enel Foundation, 00198, Viale Regina Margherita n. 137, Rome, Italy 40 3: School of Environment, Beijing Normal University, China, 北京市海淀区新街口外 41 大街19号 邮政编码: 100875 42 4: Department of Energy and Environment, TERI University, 10 Institutional Area, 43 Vasant Kunj, New Delhi, DL 110070, India 44 5: Department of Industrial Engineering, De La Salle University, 2401 Taft Ave, Malate, 45 Manila, 1004 Metro Manila, Philippines 1 46 6: Department of Landscape and Ecological Planning, Seoul National University, 1 47 Gwanak-ro, Gwanak-gu, Seoul, South Korea 48 7: COPPE, Federal University of Rio de Janeiro, Av. Pedro Calmon, 550 - Cidade 49 Universitária, Rio de Janeiro - RJ, 21941-901, Brazil 50 8: UCLA Institute of the Environment and Sustainability, La Kretz Hall, Suite 300, Los 51 Angeles, CA 90095-1496. -

February 2, 2021 Gemtier Medical (Shanghai)
February 2, 2021 Gemtier Medical (Shanghai) Inc ℅ Julie Chen Technical Manager Shanghai Medical Business Consulting Co.,Ltd. No. 170 Huajiang Road, Jiading District Shanghai, 201803 China Re: K202331 Trade/Device Name: Medical Face Mask Regulation Number: 21 CFR 878.4040 Regulation Name: Surgical Apparel Regulatory Class: Class II Product Code: FXX Dated: December 24, 2020 Received: January 6, 2021 Dear Julie Chen: We have reviewed your Section 510(k) premarket notification of intent to market the device referenced above and have determined the device is substantially equivalent (for the indications for use stated in the enclosure) to legally marketed predicate devices marketed in interstate commerce prior to May 28, 1976, the enactment date of the Medical Device Amendments, or to devices that have been reclassified in accordance with the provisions of the Federal Food, Drug, and Cosmetic Act (Act) that do not require approval of a premarket approval application (PMA). You may, therefore, market the device, subject to the general controls provisions of the Act. Although this letter refers to your product as a device, please be aware that some cleared products may instead be combination products. The 510(k) Premarket Notification Database located at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm identifies combination product submissions. The general controls provisions of the Act include requirements for annual registration, listing of devices, good manufacturing practice, labeling, and prohibitions against misbranding and adulteration. Please note: CDRH does not evaluate information related to contract liability warranties. We remind you, however, that device labeling must be truthful and not misleading. -

Business Incubator of Tongji University Science Park
1. To create a knowledge-based service innovation cluster by the radiation effect of the Tongji knowledge-based economic circle. 2. To boost the development of joint production and Business Incubator of Tongji research and construct a chain-wide innovation and start-up service system. University Science Park 3. To build a dream together with entrepreneurs by the concept of seamless collaboration team service. Location: Yangpu District, Shanghai General Introduction Address: Room 105, 65 Chifeng Road, Yangpu District, Shanghai Business Incubator of Tongji University Science Park Number of Incubated foreign invested enterprises, was established in December 2003, located in Tongji joint ventures or overseas talent startups: 30 University South Campus in Chifeng Road. It is the national Website: www.tj-ibi.com high-tech business service center and vice president unit of Major industries: Modern design, energy saving and Shanghai Science and Technology Incubator Association. environmental protection, electronic With more than 1,400 registered enterprises reside in information, rail transportation,etc. incubation sites, it covers an incubation area of 20,000 square meters. As the incubation platform of Tongji University National University Science and Technology Park, the company is Results list specialized in the incubation of small and medium enterprises of science and technology, providing the enterprises residence services and business incubation services. Performance List 55 enterprises in the park have been identified as high-tech enterprises, 9 identified as the Shanghai Little Giant Cultivating Enterprises, 4 as Shanghai Little Giant Enterprises, and 16 as Yangpu District Little Giant Enterprises. Resident enterprises have over 1000 intellectual property rights. There are 3 companies have been listed on the NEEQ and 1 company has been listed in the Shanghai Equity Trading Center. -

Arizona Governor's Office Delegation Visit to Shanghai Women's
Arizona Governor’s Office Delegation Visit to Shanghai Women’s Federation Itinerary Friday, May 15 10:30 Arrival at Pudong International Airport, Flight OZ361 11:20 Travel via Maglev train to Longyang Station Luggage van travels directly to the Park Hotel 12:10 Travel via van to Park Hotel 13:00 Hotel check-in 13:30 Free time 18:00 Depart for Art and Shanghai 18:30 Welcome Reception 20:30 Travel back to hotel Saturday, May 16 08:00 Breakfast 09:20 Depart for Shanghai Urban Planning Exhibition Center 09:30 Visit Shanghai Urban Planning Exhibition Center 10:30 Depart for Huaihai Road 10:45 Visit Exhibition Center of World Expo 2010 Shanghai 11:45 Lunch 13:00 Visit Xin Tian Di (a fashionable pedestrian street composed of Shikumen and modern architecture) 14:00 Depart for Pudong New Area 14:30 Visit Orient Pearl TV Tower and Shanghai History Museum 16:00 Shopping 18:00 Dinner 19:00 Walk on Riverside Avenue with night view of the Bund 20:00 Travel back to hotel Sunday, May 17 08:00 Breakfast 09:00 Depart for Yuyuan Garden 09:30 Visit Yuyuan Garden 10:30 Shopping 12:00 Lunch 13:30 Travel back to hotel 14:00 Visit Shanghai Museum Free time 19:00 Dinner Monday, May 18 07:30 Depart for Jinshan District 09:00 Tour of Fengjing Ancient Town 10:00 Meeting with the delegates from Jinshan District 1. Welcome speech by the leader of Jinshan District 2. Brief introduction of the work of the Jinshan Women’s Federation 3. -

OFFSHORE SCHOOL LIST International Education Branch As of August 2013
Ministry of Education OFFSHORE SCHOOL LIST International Education Branch as of August 2013 __________________________________________________________________________________ BC Global Education Program – Offshore Schools British Columbia (BC) offshore schools are inspected and certified by the BC Ministry of Education. Students registered at a BC offshore school receive instruction in English by BC certified teachers. Graduates from a BC Graduation Program are issued a BC Certificate of Graduation (Dogwood Diploma). BC Global Education Program – Offshore Schools SCHOOL NAME LOCATION OWNER/OPERATOR MINISTRY LIAISON Seongnam, Gyeonggi Dong Young Seo Larry Simpson BIS Canada Province, South Korea BIC Canada [email protected] British Columbia Canadian Karim Mostafa Margaret Compo Cairo, Egypt International School (BCCIS) BCCIS [email protected] Go In Gyung Scott Reid BC Collegiate Canada Seoul, South Korea BCA Canada Inc. [email protected] British Columbia Dr. Pakdee Tharnpanya Rodger Lindstrom International School, Bangkok, Thailand BC International School of Bangkok [email protected] Bangkok Sunny Bai Canada Changchun Shiyi Changchun, Jilin Rodger Lindstrom Beijing Kezhi Times International Secondary School Province, PRC [email protected] Consulting Co. Ltd. Sunny Bai Canada Chengdu Shi Shi Chengdu, Sichuan Rodger Lindstrom Beijing Kezhi Times International Secondary School Province, PRC [email protected] Consulting Co. Ltd. Sunny Bai Canada Hefei No. 1 Hefei, Anhui Province Rodger Lindstrom Beijing Kezhi Times International Secondary School PRC [email protected] Consulting Co. Ltd. Sunny Bai Canada Kunming No. 10 Kunming, Yunnan Rodger Lindstrom Beijing Kezhi Times International Secondary School Province, PRC [email protected] Consulting Co. Ltd. Sunny Bai Canada Langfang Langfang, Hebei Rodger Lindstrom Beijing Kezhi Times International Secondary School Province, PRC [email protected] Consulting Co. -

Download Article (PDF)
International Conference on Global Economy, Commerce and Service Science (GECSS 2014) A Study on Equalization of Basic Medical Services in Shanghai Based on Factor Analysis Yadan Zhang Chunfang Cai*, Xinde Chen* School of Management School of Management Shanghai University of Engineering Science Shanghai University of Engineering Science Shanghai, China Shanghai, China [email protected] *Corresponding author Abstract —Purpose: This paper comprehensively evaluates whether the districts in Shanghai realize the equalization of basic II. CONSTRUCTION OF FACTOR ANALYSIS MODEL medical services and aims at providing the reference for the health planning. Approaches: The author adopts SPSS software A. Construction of Index System to conduct factor analysis on each index. Results: The districts In accordance with the design of the indexes of equalization with satisfying equalization of basic medical services concentrate of basic medical services in Shanghai, the author selects 15 in the central urban areas, among which Jing’an District, Xuhui most typical indexes for the assessment and analysis of the District, Huangpu District and Changning District have the best equalization of basic medical services in the districts in equalization. Besides, there is obvious non-equalization in the Shanghai [3]. They are respectively: districts far away from the central urban area. Among them, Qingpu District, Chongming District, Jinshan District and x1→the number of health institutions for each ten Jiading District have the most serious non-equalization status. thousand people Conclusion: Generally speaking, the non-equalization of basic x2→the number of beds for each ten thousand people medical services in Shanghai is quite unsatisfying. x3→the number of health technicians for each ten Keywords- basic medical services; equalization; factor analysis thousand people x4→the number of doctors for each ten thousand people I.