Interim Report 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Chinese Pharmaceutical Startup Acquires an American Firm to “Go Global”
Paulson Papers on Investment Case Study Series A Chinese Pharmaceutical Startup Acquires an American Firm to “Go Global” June 2016 Paulson Papers on Investment Case Study Series Preface or decades, bilateral investment manufacturing—to identify tangible has flowed predominantly from the opportunities, examine constraints and FUnited States to China. But Chinese obstacles, and ultimately fashion sensible investments in the United States have investment models. expanded considerably in recent years, and this proliferation of direct investments Most of the case studies in this Investment has, in turn, sparked new debates about series look ahead. For example, our the future of US-China economic relations. agribusiness papers examine trends in the global food system and specific US and Unlike bond holdings, which can be Chinese comparative advantages. They bought or sold through a quick paper propose prospective investment models. transaction, direct investments involve people, plants, and other assets. They are But even as we look ahead, we also a vote of confidence in another country’s aim to look backward, drawing lessons economic system since they take time from past successes and failures. And both to establish and unwind. that is the purpose of the case studies, as distinct from the other papers in this The Paulson Papers on Investment aim series. Some Chinese investments in to look at the underlying economics— the United States have succeeded. They and politics—of these cross-border created or saved jobs, or have proved investments between the United States beneficial in other ways. Other Chinese and China. investments have failed: revenue sank, companies shed jobs, and, in some Many observers debate the economic, cases, businesses closed. -

The World Bank for OFFICIAL USE ONLY
Document of The World Bank FOR OFFICIAL USE ONLY Public Disclosure Authorized Report No: 49565-CN PROJECT APPRAISAL DOCUMENT ON A Public Disclosure Authorized PROPOSED GRANT FROM THE GLOBAL ENVIR0NMEN.T FACILITY TRUST FUND IN THE AMOUNT OF US$4.788 MILLION TO THE PEOPLE’S REPUBLIC OF CHINA FOR A Public Disclosure Authorized SHANGHAI AGRICULTURAL AND NON-POINT POLLUTION REDUCTION PROJECT May 18,2010 China and Mongolia Sustainable Development Unit Sustainable Development Department East Asia and Pacific Region This document has a restricted distribution and may be used by recipients only in the Public Disclosure Authorized performance of their official duties. Its contents may not otherwise be disclosed without World Bank authorization. CURRENCY EQUIVALENTS (Exchange Rate Effective September 29, 2009) Currency Unit = Renminbi Yuan (RMB) RMB6.830 = US$1 US$0.146 = RMB 1 FISCAL YEAR January 1 - December31 ABBREVIATIONS AND ACRONYMS APL Adaptable Program Loan AMP Abbreviated Resettlement Action Plan BOD Biological Oxygen Demand CAS Country Assistance Strategy CDM Clean Development Mechanism CEA Consolidated Project- Wide Environmental Assessment CEMP Consolidated Project- Wide Environmental Management Plan CNAO China National Audit Office COD Chemical Oxygen Demand CSTR Completely Stirred Tank Reactor DA Designated Account EA Environmental Assessment ECNU East China Normal University EIRR Economic Internal Rate of Return EMP Environmental Management Plan ER Emission Reduction FA0 Food and Agricultural Organization FM Financial Management FMM -

Press Release
PRESS RELEASE Merlin Entertainments announces LEGOLAND Shanghai Resort is anticipated to open in 2024 Construction of one of the largest LEGOLAND parks in the world expected to begin in 2021 6 November 2020: Merlin Entertainments (“Merlin” or “the Company”), a global leader in location based entertainment with brands including LEGOLAND®, Madame Tussauds and the Dungeons, today announces that it has entered into a formal co-operation agreement with the Shanghai Jinshan District Government, CMC Inc. and KIRKBI to develop a LEGOLAND® Resort in the Jinshan District of Shanghai, China. This follows the signing of a framework agreement in November 2019, announced as part of the China International Import Expo. Under the terms of the agreement, all parties will form a joint venture company and contribute funding to the construction and development of LEGOLAND® Shanghai. The total project investment is expected to be approximately $550 million. Construction of the project is planned to start next year, and the Resort is expected to open in 2024. LEGOLAND® Shanghai will be one of the largest LEGOLAND® Resorts in the world and will incorporate a 250-room fully themed hotel on opening. World-leading creative, design and construction teams will work together to create an immersive theme park, drawing inspiration from famous scenic spots in Shanghai, Jinshan District and the town of Fengjing. It will be located in the Jinshan District in south west Shanghai with a two- hour catchment of 55 million people. The region comprising Shanghai, Jiangsu, Zhejiang and Anhui has an estimated population of 220 million. China is a focus of significant development and investment by Merlin. -

Heng Feng Road, Zhabei District, Shanghai, China
Heng Feng Road, Zhabei District, Shanghai, China View this office online at: https://www.newofficeasia.com/details/offices-heng-feng-road-zhabei-district- shanghai This fully serviced business centre is in a great location within a premium office building offering spectacular views of the Su Zhou Creek. There's a comprehensive package of services available for clients, including IT support, accounting assistance and business licencing. There are conference rooms available, a telephone answering service and other types of administrative support, all from a highly convenient town centre location offering 24 hour access, security system and plenty of car parking spaces. Transport links Nearest tube: Metro Line 1, Han Zhong Road Station Nearest railway station: Shanghai Railway Station Nearest road: Metro Line 1, Han Zhong Road Station Nearest airport: Metro Line 1, Han Zhong Road Station Key features 24 hour access Access to multiple centres nation-wide Administrative support Car parking spaces Close to railway station Conference rooms Conference rooms High speed internet IT support available Meeting rooms Modern interiors Near to subway / underground station Reception staff Security system Telephone answering service Town centre location Location This business centre is in a great location in the central business district amongst the hub of public transportation choices. It's only 50 metres from Subway Line 1, alongside Huaihai Road and Nanjing Road and Shanghai Railway Station is also easily accessible. Points of interest within 1000 metres Hanzhong -

The Oriental Pearl Radio & TV Tower 东方明珠 Getting in Redeem Your
The Oriental Pearl Radio & TV Tower 东方明珠 Getting In Redeem your pass for an admission ticket at the first ticket office, near No. 1 Gate. Hours Daily, 8:00 am-9:30 pm. Address No. 1 Lujiazui Century Ave Pudong New Area, Shanghai Public Transportation Take Metro Line 2 and get off at Lujiazui Station, get out from Exit 1 and walk to The Oriental Pearl Radio & TV Tower. Yu Garden (Yuyuan) 豫园 Getting In Please redeem your pass for an admission ticket at the Yuyuan Garden ticket office located on the north side of the Huxin Pavilion Jiuqu Bridge prior to entry. Hours Daily, 8:45 am-4:45 pm. Address No. 218 Anren St Huangpu District, Shanghai Public Transportation Take Metro Line 10 and get off at Yuyuan Station, then walk to Yu Garden. Shanghai World Financial Center Observatory 上海环球金融中心 Getting In Please redeem your pass for an admission ticket at the Global Finance Center F1 ticket window located at Lujiazui Century Ave. Hours Daily, 9:00 am-10:30 pm. Address B1 Ticketing Window, World Financial Center 100 Century Avenue Lujiazui, Pudong New Area, Shanghai Public Transportation Take Metro Line 2 and get off at Lujiazui Station, then walk to Shanghai World Financial Center. Shanghai Hop-On Hop-Off Sightseeing Bus Tour 观光巴士 Getting In You must first redeem your pass for a bus ticket at one of the following locations prior to boarding: Nanjing Road Station (New World City Stop): Opposite to New World City, No. 2-88 Nanjing West Road, Huangpu District, Shanghai Bund A Station (Sanyang Food Stop): Beside Sanyang Food, 367 East Zhongshan Road, Huangpu District, Shanghai (near Beijing East Road) Shiliupu Station (Pujiang Tour Terminal Stop): 531 Zhongshan East Second Road, Huangpu District, Shanghai Yuyuan Station (Yongan Road, Renmin Road): Xinkaihe Road, Renmin Road, next to the bus stop in front of the Bund soho. -
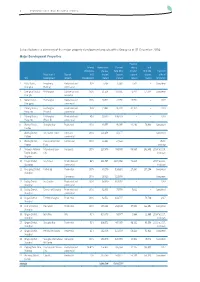
Major Development Properties
1 SHANGHAI INDUSTRIAL HOLDINGS LIMITED Set out below is a summary of the major property development projects of the Group as at 31 December 2016: Major Development Properties Pre-sold Interest Approximate Planned during Total attributable site area total GFA the year GFA sold Expected Projects of SI Type of to SI (square (square (square (square date of City Development property Development meters) meters) meters) meters) completion 1 Kaifu District, Fengsheng Residential and 90% 5,468 70,566 7,542 – Completed Changsha Building commercial 2 Chenghua District, Hi-Shanghai Commercial and 100% 61,506 254,885 75,441 151,644 Completed Chengdu residential 3 Beibei District, Hi-Shanghai Residential and 100% 30,845 74,935 20,092 – 2019 Chongqing commercial 4 Yuhang District, Hi-Shanghai Residential and 85% 74,864 230,484 81,104 – 2019 Hangzhou (Phase I) commercial 5 Yuhang District, Hi-Shanghai Residential and 85% 59,640 198,203 – – 2019 Hangzhou (Phase II) commercial 6 Wuxing District, Shanghai Bay Residential 100% 85,555 96,085 42,236 76,966 Completed Huzhou 7 Wuxing District, SIIC Garden Hotel Hotel and 100% 116,458 47,177 – – Completed Huzhou commercial 8 Wuxing District, Hurun Commercial Commercial 100% 13,661 27,322 – – Under Huzhou Plaza planning 9 Shilaoren National International Beer Composite 100% 227,675 783,500 58,387 262,459 2014 to 2018, Tourist Resort, City in phases Qingdao 10 Fengze District, Sea Palace Residential and 49% 381,795 1,670,032 71,225 – 2017 to 2021, Quanzhou commercial in phases 11 Changning District, United 88 Residential -

Report on the Parliamentary Trade Mission to Shanghai Honourable
Report on the Parliamentary Trade Mission to Shanghai Honourable Curtis Pitt MP Speaker of the Legislative Assembly 21 -27 September 2019 1 TABLE OF CONTENTS EXECUTIVE SUMMARY ................................................................................... 3 OBJECTIVES OF THE QUEENSLAND PARLIAMENTARY TRADE DELEGATION ..... 4 QUEENSLAND – CHINA RELATIONSHIP ........................................................... 5 MISSION DELEGATION MEMBERS .................................................................. 9 PROGRAM ................................................................................................... 10 RECPEPTION: QUEENSLAND YOUTH ORCHESTRA ENSEMBLE PERFORMANCE AND DINNER WITH QUEENSLAND DELEGATES ............................................. 21 MEETING: BUNDABERG BREWED DRINKS .................................................... 23 MEETING: AUSTCHAM SHANGHAI ............................................................... 25 MEETING: SHANGHAI PEOPLE’S CONGRESS ................................................. 27 SITE VISIT: SENSETIME ................................................................................. 29 RECEPTION: QUEENSLAND GOVERNMENT RECEPTION ................................ 32 MEETING: ALIBABA GROUP .......................................................................... 34 TIQ BUSINESS DINNER ................................................................................. 40 MEETING: JINSHAN DISTRICT PEOPLE’S CONGRESS ...................................... 41 SITE VISIT: FENGJING ANCIENT TOWN, -

The Oriental Pearl Radio & TV Tower 东方明珠
The Oriental Pearl Radio & TV Tower 东方明珠 Hours: Daily, 9:00 am-9:30 pm. Address: No. 1 Century Ave Pudong New Area (Lujiazui), Shanghai Public Transportation Take Metro Line 2 and get off at Lujiazui Station, get out from Exit 1 and walk to The Oriental Pearl Radio & TV Tower. Getting In Redeem your pass for an admission ticket at the first ticket office, near No. 1 Gate: Shanghai World Financial Center Observatory 上海环球金融中心 Hours: Daily, 9:00 am-10:00 pm. Address: B1 Ticketing Window, World Financial Center 100 Century Avenue Lujiazui, Pudong New Area, Shanghai Public Transportation Take Metro Line 2 and get off at Lujiazui Station, then walk to Shanghai World Financial Center. Getting In Please redeem your pass for an admission ticket at B1 Ticketing Window, World Financial Center at Lujiazui Century Ave: Pujiang River Cruise Tour 黄浦江“清游江”游览船 Hours:Daily, 10:00 am-8:30 pm. Address:Shiliupu Cruise Terminal,No. 481 Zongshan Rd,Huangpu District, Shanghai Public Transportation Bus: Take the bus #33, 55, 65, 305, 868, 910, 926 or 928 and get off at the Xinkaihe Road-Bus Stop of Zhongshan East Second Road, then walk to No. 481, Zhongshan East Second Road, Huangpu District. Getting In Redeem your pass for an admission ticket at the Shiliu Pu Pier, Huangpu River Tour ticket window at 481 Zhongshan 2nd Rd: Yu Garden (Yuyuan) 豫园 Hours: Daily, 8:45 am-4:45 pm. Address: No. 218 Anren St Huangpu District, Shanghai Public Transportation Take Metro Line 10 and get off at Yuyuan Station, then walk to Yu Garden. -
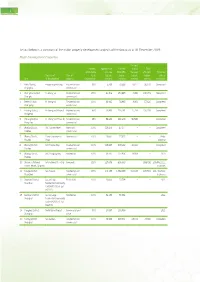
Set out Below Is a Summary of the Major Property Development Projects of the Group As at 31 December 2019: Major Development
1 Set out below is a summary of the major property development projects of the Group as at 31 December 2019: Major Development Properties Pre-sold Interest Approximate Planned during Total attributable site area total GFA the year GFA sold Expected Projects of Type of to SI (square (square (square (square date of City SI Development property Development meters) meters) meters) meters) completion 1 Kaifu District, Fengsheng Building Residential and 90% 5,468 70,566 6,627 30,870 Completed Changsha commercial 2 Chenghua District, Hi-Shanghai Residential and 100% 61,506 254,885 4,996 190,153 Completed Chengdu commercial 3 Beibei District, Hi-Shanghai Residential and 100% 30,845 74,935 3,301 57,626 Completed Chongqing commercial 4 Yuhang District, Hi-Shanghai (Phase I) Residential and 85% 74,864 230,484 27,758 150,289 Completed Hangzhou commercial 5 Yuhang District, Hi-Shanghai (Phase II) Residential and 85% 59,640 198,203 56,539 – Completed Hangzhou commercial 6 Wuxing District, SIIC Garden Hotel Hotel and 100% 116,458 47,177 – – Completed Huzhou commercial 7 Wuxing District, Hurun Commercial Commercial 100% 13,661 27,322 – – Under Huzhou Plaza planning 8 Wuxing District, SIIC Tianlan Bay Residential and 100% 115,647 193,292 26,042 – Completed Huzhou commercial 9 Wuxing District, SIIC Yungjing Bay Residential 100% 68,471 207,906 28,953 – 2020 Huzhou 10 Shilaoren National International Beer City Composite 100% 227,675 806,339 – 333,798 2014 to 2022, Tourist Resort, Qingdao in phases 11 Fengze District, Sea Palace Residential and 100% 170,133 -

Pacer CSOP FTSE China A50 ETF Schedule of Investments July 31, 2020 (Unaudited) Shares Value COMMON STOCKS - 98.0% Agriculture - 1.6% Muyuan Foodstuff Co Ltd
Page 1 of 4 Pacer CSOP FTSE China A50 ETF Schedule of Investments July 31, 2020 (Unaudited) Shares Value COMMON STOCKS - 98.0% Agriculture - 1.6% Muyuan Foodstuff Co Ltd. - Class A 9,230 $ 120,977 Wens Foodstuffs Group Co Ltd. - Class A 4,660 15,857 136,834 Auto Manufacturers - 0.7% SAIC Motor Corp Ltd. - Class A 24,600 64,077 Banks - 23.7% Agricultural Bank of China Ltd. - Class H 352,300 163,039 Bank of China Ltd. - Class H 193,900 92,512 Bank of Communications Co Ltd. - Class A 184,100 125,556 China CITIC Bank Corp Ltd. - Class H 24,700 18,261 China Construction Bank Corp. - Class H 81,500 71,464 China Everbright Bank Co Ltd. - Class H 126,400 68,456 China Merchants Bank Co Ltd. - Class A 108,200 539,489 China Minsheng Banking Corp Ltd. - Class A 254,300 201,851 Industrial & Commercial Bank of China Ltd. - Class A 198,400 140,993 Industrial Bank Co Ltd. - Class A 127,400 285,849 Ping An Bank Co Ltd. - Class A 75,000 143,348 Shanghai Pudong Development Bank Co Ltd. - Class A 132,300 196,379 2,047,197 Beverages - 17.9% Jiangsu Yanghe Brewery Joint-Stock Co Ltd. - Class A 4,000 77,398 Kweichow Moutai Co Ltd. - Class A 4,000 961,777 Wuliangye Yibin Co Ltd. - Class A 16,200 504,835 1,544,010 Building Materials - 1.6% Anhui Conch Cement Co Ltd. - Class H 15,900 139,921 Coal - 0.5% China Shenhua Energy Co Ltd. -

Schedule of Investments (Unaudited) Ishares MSCI Total International Index Fund (Percentages Shown Are Based on Net Assets) September 30, 2020
Schedule of Investments (unaudited) iShares MSCI Total International Index Fund (Percentages shown are based on Net Assets) September 30, 2020 Mutual Fund Value Total International ex U.S. Index Master Portfolio of Master Investment Portfolio $ 1,034,086,323 Total Investments — 100.4% (Cost: $929,170,670) 1,034,086,323 Liabilities in Excess of Other Assets — (0.4)% (3,643,126) Net Assets — 100.0% $ 1,030,443,197 iShares MSCI Total International Index Fund (the “Fund”) seeks to achieve its investment objective by investing all of its assets in International Tilts Master Portfolio (the “Master Portfolio”), which has the same investment objective and strategies as the Fund. As of September 30, 2020, the value of the investment and the percentage owned by the Fund of the Master Portfolio was $1,034,086,323 and 99.9%, respectively. The Fund records its investment in the Master Portfolio at fair value. The Fund’s investment in the Master Portfolio is valued pursuant to the pricing policies approved by the Board of Directors of the Master Portfolio. Fair Value Hierarchy as of Period End Various inputs are used in determining the fair value of financial instruments. These inputs to valuation techniques are categorized into a fair value hierarchy consisting of three broad levels for financial reporting purposes as follows: • Level 1 – Unadjusted price quotations in active markets/exchanges for identical assets or liabilities that the Fund has the ability to access • Level 2 – Other observable inputs (including, but not limited to, quoted prices -

Development of High-Speed Rail in the People's Republic of China
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Haixiao, Pan; Ya, Gao Working Paper Development of high-speed rail in the People's Republic of China ADBI Working Paper Series, No. 959 Provided in Cooperation with: Asian Development Bank Institute (ADBI), Tokyo Suggested Citation: Haixiao, Pan; Ya, Gao (2019) : Development of high-speed rail in the People's Republic of China, ADBI Working Paper Series, No. 959, Asian Development Bank Institute (ADBI), Tokyo This Version is available at: http://hdl.handle.net/10419/222726 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. may exercise further usage rights as specified in the indicated licence. https://creativecommons.org/licenses/by-nc-nd/3.0/igo/ www.econstor.eu ADBI Working Paper Series DEVELOPMENT OF HIGH-SPEED RAIL IN THE PEOPLE’S REPUBLIC OF CHINA Pan Haixiao and Gao Ya No.