Review Article Single-Stranded DNA Aptamers Against Pathogens and Toxins: Identification and Biosensing Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FOLIA VETERINARIA Is a Scientific Journal Issued by the University of Veterinary Medicine and Pharmacy in Košice, Komenského 73, 041 81 Košice, Slovakia
FOLIA VETERINARIA The scientific journal of the ISSN 0015-5748 UNIVERSITY OF VETERINARY MEDICINE AND eISSN PHARMACY IN KOŠICE — Slovakia 2453-7837 2 LXIV • 2020 FOLIA VETERINARIA is a scientific journal issued by the University of Veterinary Medicine and Pharmacy in Košice, Komenského 73, 041 81 Košice, Slovakia. The journal is published quaterly in English (numbers 1—4) and distributed worldwide. The list of Editorial Board of scientific journal Folia Veterinaria: Editor-in-Chief: Jana Mojžišová Deputy/Managing Editor: Juraj Pistl Editorial Board: Aland, A. (Tartu, Estonia), Banhazi, T. (Toowomba, Aus- tralia), Bao, E. (Nanjing, China), Bíreš, J. (Bratislava, Slovakia), Celer, V. (Brno, Czechia), Fablet, Ch. (Ploufragan, France), Faix, Š. (Košice, Slovakia), Faixová, Z. (Košice, Slovakia), Fedoročko, P. (Košice, Slovakia), Gunnarsson, S. (Skara, Sweden), Kolacz, R. (Wrocław, Poland), Könyves, L. (Budapest, Hungary), Nagy, J. (Košice, Slovakia), Novák, M. (Bratislava, Slovakia), Paulsen, P. (Vienna, Austria), Pěchová, A. (Brno, Czechia), Sossidou, E. N. (Thermi Thessa- loniki, Greece), Večerek, V. (Brno, Czechia), Vorlová, V. (Brno, Czechia) Vargová, M. — technical editor (Košice, Slovakia) Contact: tel.: +421 915 984 669 e-mail: [email protected] Electronic Publisher: De Gruyter Poland, Bogumila Zuga 32A 01-811 Warsaw, Poland ISSN 2453-7837 on-line ISSN 0015-5748 print EV 3485/09 Publisher’s Identification number: IČO 00397474 June 2020 FOLIA VETERINARIA PUBLISHED BY THE UNIVERSITY OF VETERINARY MEDICINE AND PHARMACY IN KOŠICE SLOVAK IA Folia Veterinaria Vol. 64, 2, 2020 V YDÁVA UNIVERZITA VETERINÁRSKEHO LEKÁRSTVA A FARMÁCIE V KOŠICIACH 2020 F O L I A V E T E R I N A R I A, 64, 2, 2020 C O N T E N T S ADESOKAN, H. -

Evaluation of the Individual and Combined Toxicity of Fumonisin Mycotoxins in Human Gastric Epithelial Cells
International Journal of Molecular Sciences Article Evaluation of the Individual and Combined Toxicity of Fumonisin Mycotoxins in Human Gastric Epithelial Cells Song Yu, Bingxuan Jia, Na Liu, Dianzhen Yu and Aibo Wu * SIBS-UGENT-SJTU Joint Laboratory of Mycotoxin Research, CAS Key Laboratory of Nutrition, Metabolism and Food Safety, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200031, China; [email protected] (S.Y.); [email protected] (B.J.); [email protected] (N.L.); [email protected] (D.Y.) * Correspondence: [email protected]; Tel.: +86-21-54920716 Received: 23 July 2020; Accepted: 14 August 2020; Published: 18 August 2020 Abstract: Fumonisin contaminates food and feed extensively throughout the world, causing chronic and acute toxicity in human and animals. Currently, studies on the toxicology of fumonisins mainly focus on fumonisin B1 (FB1). Considering that FB1, fumonisin B2 (FB2) and fumonisin B3 (FB3) could coexist in food and feed, a study regarding a single toxin, FB1, may not completely reflect the toxicity of fumonisin. The gastrointestinal tract is usually exposed to these dietary toxins. In our study, the human gastric epithelial cell line (GES-1) was used as in vitro model to evaluate the toxicity of fumonisin. Firstly, we found that they could cause a decrease in cell viability, and increase in membrane leakage, cell death and the induction of expression of markers for endoplasmic reticulum (ER) stress. Their toxicity potency rank is FB1 > FB2 >> FB3. The results also showed that the synergistic effect appeared in the combinations of FB1 + FB2 and FB1 + FB3. -

| Hao Wanatha Maria Del Contatta Datum
|HAO WANATHA MARIAUS009844679B2 DEL CONTATTA DATUM (12 ) United States Patent ( 10 ) Patent No. : US 9 ,844 , 679 B2 Nayfach - Battilana ( 45 ) Date of Patent : * Dec . 19 , 2017 (54 ) NANOPARTICLE - SIZED MAGNETIC (56 ) References Cited ABSORPTION ENHANCERS HAVING THREE - DIMENSIONAL GEOMETRIES U . S . PATENT DOCUMENTS ADAPTED FOR IMPROVED DIAGNOSTICS 4 , 106 ,488 A 8 / 1978 Gordon AND HYPERTHERMIC TREATMENT 4 ,303 ,636 A 12/ 1981 Gordon ( 71 ) Applicant: Qteris, Inc. , San Rafael, CA (US ) ( Continued ) ( 72 ) Inventor : Joseph N . Nayfach - Battilana , San FOREIGN PATENT DOCUMENTS Rafael , CA (US ) EP 0040512 B1 11 / 1981 EP 0136530 A16 /1988 (73 ) Assignee : Qteris, Inc. , San Rafael , CA (US ) (Continued ) ( * ) Notice : Subject to any disclaimer , the term of this patent is extended or adjusted under 35 OTHER PUBLICATIONS U . S . C . 154 ( b ) by 903 days. “ Krishna et al ., Unusual size -dependent magnetization in near This patent is subject to a terminal dis hemispherical Co nanomagnets on SiO . sub . 2 from fast pulsed laser claimer . processing” , J . Appl. Phys. 103 , 073902 (2008 ) (“ Krishna ” ) .* (Continued ) ( 21 ) Appl . No .: 14 /044 , 251 Primary Examiner — Joseph Stoklosa (22 ) Filed : Oct. 2 , 2013 Assistant Examiner — Adam Avigan (74 ) Attorney , Agent, or Firm — Marek Alboszta (65 ) Prior Publication Data US 2014 /0172049 A1 Jun . 19 , 2014 (57 ) ABSTRACT Nanoparticle- sized magnetic absorption enhancers (MAES ) Related U . S . Application Data exhibiting a controlled response to a magnetic field , includ ing a controlled mechanical response and an inductive (62 ) Division of application No . 12 /925 ,904 , filed on Nov. thermal response . The MAEs have a magnetic material that 1 , 2010 , now Pat . -

Rope Parasite” the Rope Parasite Parasites: Nearly Every Au�S�C Child I Ever Treated Proved to Carry a Significant Parasite Burden
Au#sm: 2015 Dietrich Klinghardt MD, PhD Infec4ons and Infestaons Chronic Infecons, Infesta#ons and ASD Infec4ons affect us in 3 ways: 1. Immune reac,on against the microbes or their metabolic products Treatment: low dose immunotherapy (LDI, LDA, EPD) 2. Effects of their secreted endo- and exotoxins and metabolic waste Treatment: colon hydrotherapy, sauna, intes4nal binders (Enterosgel, MicroSilica, chlorella, zeolite), detoxificaon with herbs and medical drugs, ac4vaon of detox pathways by solving underlying blocKages (methylaon, etc.) 3. Compe,,on for our micronutrients Treatment: decrease microbial load, consider vitamin/mineral protocol Lyme, Toxins and Epigene#cs • In 2000 I examined 10 au4s4c children with no Known history of Lyme disease (age 3-10), with the IgeneX Western Blot test – aer successful treatment. 5 children were IgM posi4ve, 3 children IgG, 2 children were negave. That is 80% of the children had clinical Lyme disease, none the history of a 4cK bite! • Why is it taking so long for au4sm-literate prac44oners to embrace the fact, that many au4s4c children have contracted Lyme or several co-infec4ons in the womb from an oVen asymptomac mother? Why not become Lyme literate also? • Infec4ons can be treated without the use of an4bio4cs, using liposomal ozonated essen4al oils, herbs, ozone, Rife devices, PEMF, colloidal silver, regular s.c injecons of artesunate, the Klinghardt co-infec4on cocKtail and more. • Symptomac infec4ons and infestaons are almost always the result of a high body burden of glyphosate, mercury and aluminum - against the bacKdrop of epigene4c injuries (epimutaons) suffered in the womb or from our ancestors( trauma, vaccine adjuvants, worK place related lead, aluminum, herbicides etc., electromagne4c radiaon exposures etc.) • Most symptoms are caused by a confused upregulated immune system (molecular mimicry) Toxins from a toxic environment enter our system through damaged boundaries and membranes (gut barrier, blood brain barrier, damaged endothelium, etc.). -

Investigation of Highly-Reducing Polyketide Synthase Enzymes That Produce the Fungal Polyketides Lovastatin, Fumonisin Bj, and Hypothemycin
University of Alberta Investigation of Highly-Reducing Polyketide Synthase Enzymes that Produce the Fungal Polyketides Lovastatin, Fumonisin Bj, and Hypothemycin by Jesse W.-H Li A thesis submitted to the Faculty of Graduate Studies and Research in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Chemistry © Li, W.-H. Jesse Spring, 2011 Edmonton, Alberta Permission is hereby granted to the University of Alberta Libraries to reproduce single copies of this thesis and to lend or sell such copies for private, scholarly or scientific research purposes only. Where the thesis is converted to, or otherwise made available in digital form, the University of Alberta will advise potential users of the thesis of these terms. The author reserves all other publication and other rights in association with the copyright in the thesis and, except as herein before provided, neither the thesis nor any substantial portion thereof may be printed or otherwise reproduced in any material form whatsoever without the author's prior written permission. Library and Archives Bibliotheque et Canada Archives Canada Published Heritage Direction du Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A0N4 Ottawa ON K1A 0N4 Canada Canada Your file Votre reference ISBN: 978-0-494-87888-0 Our file Notre reference ISBN: 978-0-494-87888-0 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library and permettant a la Bibliotheque et Archives Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par I'lnternet, preter, telecommunication or on the Internet, distribuer et vendre des theses partout dans le loan, distrbute and sell theses monde, a des fins commerciales ou autres, sur worldwide, for commercial or non support microforme, papier, electronique et/ou commercial purposes, in microform, autres formats. -

Mycotoxin Analysis IV Section 7
OOD AND RUG DMINISTRATION Revision #: 02 F D A Document Number: OFFICE OF REGULATORY AFFAIRS Revision Date: IV-07 ORA Laboratory Manual Volume IV Section 7 05/27/2020 Title: Mycotoxin Analysis Page 1 of 25 Sections in This Document 1. Purpose ....................................................................................................................................1 2. Scope .......................................................................................................................................2 3. Responsibility............................................................................................................................2 4. Background...............................................................................................................................3 5. References ...............................................................................................................................4 6. Procedure .................................................................................................................................6 6.1. Safety Precautions .........................................................................................................6 6.2. Sample Preparation .......................................................................................................7 6.3. Exercises........................................................................................................................9 6.3.1. Aflatoxins B1, B2, G1, and G2 ..........................................................................9 -

Promising Detoxification Strategies to Mitigate Mycotoxins in Food and Feed
Promising Detoxification Strategies to Mitigate Mycotoxins in Food and Feed Edited by Ting Zhou Printed Edition of the Special Issue Published in Toxins www.mdpi.com/journal/toxins Promising Detoxification Strategies to Mitigate Mycotoxins in Food and Feed Promising Detoxification Strategies to Mitigate Mycotoxins in Food and Feed Special Issue Editor Ting Zhou MDPI • Basel • Beijing • Wuhan • Barcelona • Belgrade Special Issue Editor Ting Zhou Guelph Research and Development Center (AAFC) Canada Editorial Office MDPI St. Alban-Anlage 66 Basel, Switzerland This is a reprint of articles from the Special Issue published online in the open access journal Toxins (ISSN 2072-6651) from 2016 to 2018 (available at: http://www.mdpi.com/journal/toxins/special issues/promising detoxification) For citation purposes, cite each article independently as indicated on the article page online and as indicated below: LastName, A.A.; LastName, B.B.; LastName, C.C. Article Title. Journal Name Year, Article Number, Page Range. ISBN 978-3-03897-027-9 (Pbk) ISBN 978-3-03897-028-6 (PDF) Cover image courtesy of Yousef I. Hassan. Articles in this volume are Open Access and distributed under the Creative Commons Attribution (CC BY) license, which allows users to download, copy and build upon published articles even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. The book taken as a whole is c 2018 MDPI, Basel, Switzerland, distributed under the terms and conditions of the Creative Commons license CC BY-NC-ND (http://creativecommons.org/licenses/by-nc-nd/4.0/). -

Mycotoxins and Human Health
chapter 6. Mycotoxins and human health Summary especially in vulnerable populations. 1. Introduction For fumonisins, studies indicate a Mycotoxins have been investigated possible role in oesophageal cancer This chapter covers the effects on in relation to a wide range of adverse and in neural tube defects, although human health of the major mycotoxins human health effects, but the evidence no definitive conclusions can be occurring in foods. This chapter also for all but a small number of associations drawn at present. For deoxynivalenol includes information on mechanisms is limited. Thus, the full impact on human and other trichothecenes, exposure of action of mycotoxins in humans CHAPTER 6 CHAPTER health of the widespread exposure has been linked to acute poisoning where relevant to the adverse health to mycotoxins remains to be defined. outbreaks in large numbers of effects under consideration. No The main exception is for aflatoxins; subjects. For ochratoxin A and attempt is made at a comprehensive epidemiological, experimental, and zearalenone, the human health review, but at appropriate points we mechanistic studies have contributed effects remain undefined. The limited refer to more extensive accounts. to establishing aflatoxins as a tools available to accurately assess The major source of human cause of human liver cancer, with a human exposure to mycotoxins and exposure to mycotoxins is consump- particularly elevated risk in people the relative paucity of epidemiological tion of contaminated foods. Exposure chronically infected with hepatitis B studies need to be addressed if the full is highest when those foods are dietary virus. In addition, acute aflatoxicosis extent of the adverse effects of these staples, such as maize, groundnuts, or after exposure to high dietary toxin common dietary contaminants is to be various other cereals. -

FUMONISIN B1 1. Exposure Data
FUMONISIN B1 This substance was considered by a previous working group in June 1992 (IARC, 1993). Since that time, new data have become available, and these have been incor- porated into the monograph and taken into consideration in the present evaluation. 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Nomenclature Chem. Abstr. Serv. Reg. No.: 116355-83-0 Chem. Abstr. Serv. Name: 1,2,3-Propanetricarboxylic acid, 1,1′-[1-(12-amino-4,9,11- trihydroxy-2-methyltridecyl)-2-(1-methylpentyl)-1,2-ethanediyl] ester Synonyms: FB1; macrofusine 1.1.2 Structural and molecular formulae and relative molecular mass O COOH O CCH2 CH CH2 COOH NH2 OH OH CH3 H3CCHCHCH2 CH (CH2)4 CH CH2 CH CH2 CH CH CH (CH2)3 CH3 OH CH3 HOOC CH2 CH CH2 C O COOH O C34H59NO15 Relative molecular mass: 721 1.1.3 Chemical and physical properties of the pure substance From WHO (2000) unless otherwise noted (a) Description: White hygroscopic powder (b) Melting-point: Not known (has not been crystallized) (c) Spectroscopy: Mass spectral and nuclear magnetic resonance spectroscopy data have been reported (Bezuidenhout et al., 1988; Laurent et al., 1989a; Plattner et al., 1990; Savard & Blackwell, 1994) –301– 302 IARC MONOGRAPHS VOLUME 82 (d ) Solubility: Soluble in water to at least to 20 g/L (National Toxicology Program, 2000); soluble in methanol, acetonitrile–water (e) Octanol/water partition coefficient (log P): 1.84 (Norred et al., 1997) (f ) Stability: Stable in acetonitrile–water (1:1) at 25 °C; unstable in methanol at 25 °C, forming monomethyl or dimethyl esters (Gelderblom et al., 1992a; Visconti et al., 1994); stable in methanol at –18 °C (Visconti et al., 1994); stable at 78 °C in buffer solutions at pH between 4.8 and 9 (Howard et al., 1998) 1.1.4 Analysis Methods for the analysis of fumonisins have been extensively reviewed (WHO, 2000). -
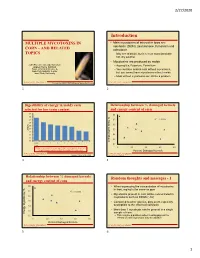
Introduction MULTIPLE MYCOTOXINS in • Main Mycotoxins of Interest in Iowa Are Vomitoxin (DON), Zearalenone, Fumonisin and CORN – and RELATED Ochratoxin
2/27/2020 Introduction MULTIPLE MYCOTOXINS IN • Main mycotoxins of interest in Iowa are vomitoxin (DON), zearalenone, fumonisin and CORN – AND RELATED ochratoxin. TOPICS – May see aflatoxin, but it is more associated with hot, dry weather • Mycotoxins are produced by molds John Patience & Leigh Ruckman – Aspergillus, Fusarium, Penicillium Applied Swine Nutrition Dept. of Animal Science & – You can have mold in corn without mycotoxins, Iowa Pork Industry Center Iowa State University but you cannot have mycotoxins without molds – Mold without mycotoxins can still be a problem IOWA STATE UNIVERSITY IOWA STATE UNIVERSITY Presented at 2020 IPPA/IPIC Regional Swine Conferences APPLIED SWINE NUTRITION APPLIED SWINE NUTRITION 1 2 Digestibility of energy in moldy corn Relationship between % damaged kernels selected for low toxin content and energy content of corn 89 88 88 87 87 R² = 0.2186 86 85 86 84 ATTD, % ATTD, 85 83 82 84 81 83 80 Energy Digestibility, % Digestibility, Energy Good 1234567 Corn 82 Moldy corn contained, on average, 3.4% less energy than clean corn. 0 20 40 60 80 The worst sample contained 5.1% less energy than clean corn. Percent Damaged Kernels IOWA STATE UNIVERSITY IOWA STATE UNIVERSITY APPLIED SWINE NUTRITION Source: Pilcher at al., 2010 APPLIED SWINE NUTRITION 3 4 Relationship between % damaged kernels Random thoughts and messages - 1 and energy content of corn 88 • When expressing the concentration of mycotoxins in feed, mg/kg is the same as ppm 87 R² = 0.5885 • Mycotoxins present in corn will be concentrated in 86 co-products -

Mycotoxins in Fruit and Fruit Products
Handling & Processing Section Proc. Fla. State Hort. Soc. 125:252–257. 2012. Mycotoxins in Fruit and Fruit Products Devin C. Lewis anD Renee GooDRiCh-sChneiDeR* University of Florida, Food Science and Human Nutrition Department, 475 FSHN Building, Newell Drive, Gainesville, FL 32611-0370 AdditionAl index words. Penicillium, Fusarium, Aspergillus, patulin, ochratoxin, fumonisin, aflatoxin, toxicity, grapes, apples, juice, beverage The term “mycotoxin” is generally associated with secondary metabolic toxic products of filamentous fungi to which humans and animals can be exposed, most often through ingestion. This exposure can result in a range of toxicities (acute to chronic), and a spectrum of effects (mild to severe) including carcinogenicity and death. The history of mycotoxicoses has been long and dramatic, including a cereal-associated outbreak of ergot contamination that is thought to have catalyzed the unfortunate events known as the Salem Witch Trials. While many significant mycotoxins are associated with grain-based food products, fruit and fruit products can also be affected. Patulin, a mycotoxin produced by many Penicillium spp., is a known hazard in the apple juice industry where its levels are under regulatory control (no more than 50 ppb in apple juice). Orange juice and fermented fruit beverages such as wine are also susceptible to mycotoxin contamination from Fusarium and Aspergillus spp., respectively. Other fruits support the growth of mycotoxin-producing fungi, although specific conditions that lead to the production of mycotoxins are not well-elucidated. Inconsistencies in reports and lack of survey data on the prevalence and level of mycotoxins in fruit products makes the risk assessment of mycotoxins in fruit products challenging. -

Dynamic Fumonisin B2 Production by Aspergillus Niger Intented Used in Food Industry in China
toxins Article Dynamic Fumonisin B2 Production by Aspergillus niger Intented Used in Food Industry in China Xiaomin Han 1,†, Hongru Jiang 2,†, Jin Xu 1, Jing Zhang 1 and Fengqin Li 1,* 1 Key Laboratory of Food Safety Risk Assessment, Ministry of Health, China National Center for Food Safety Risk Assessment, Beijing 100021, China; [email protected] (X.H.); [email protected] (J.X.); [email protected] (J.Z.) 2 National Institute for Nutrition and Health, Chinese Centre for Disease Control and Prevention, Beijing 100050, China; [email protected] * Correspondence: [email protected]; Tel./Fax: +86-10-6777-6356 † These authors contributed equally to this work. Academic Editor: Paola Battilani Received: 1 June 2017; Accepted: 6 July 2017; Published: 9 July 2017 Abstract: There are a total of 30 strains including 27 strains of Aspergillus niger intended used in Chinese food industry, two strains used as control and one strain isolated from corn for fumonisin (FB) production on 3 media. It was found that FB2 production by A. niger was function-dependent and highly related to culture media, as well as incubation time. All strains studied were unable to produce FB1 and FB3. Almost all strains were found to produce FB2 on corn, rice and wheat bran. Based on their intended use in the food industry, the higher level of FB2 producers were strains used for saccharifying enzyme (n = 13) production, followed by organic acid (n = 6), tannase (n = 7) and β-galactosidase (n = 1) production, with the FB2 mean level of 3553–10,270 µg/kg, 1059–12,036 µg/kg, 3–7 µg/kg and 2–4 µg/kg on corn, 5455–9241 µg/kg, 559–2190 µg/kg, 4–9 µg/kg and 6–10 µg/kg on rice, 5959–7709 µg/kg, 9491–17,339 µg/kg, 8–14 µg/kg and 120–222 µg/kg on wheat bran, respectively.