Evaluation of Doping in 4H-Sic by Optical Spectroscopies Pawel Kwasnicki
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DMAAC – February 1973
LUNAR TOPOGRAPHIC ORTHOPHOTOMAP (LTO) AND LUNAR ORTHOPHOTMAP (LO) SERIES (Published by DMATC) Lunar Topographic Orthophotmaps and Lunar Orthophotomaps Scale: 1:250,000 Projection: Transverse Mercator Sheet Size: 25.5”x 26.5” The Lunar Topographic Orthophotmaps and Lunar Orthophotomaps Series are the first comprehensive and continuous mapping to be accomplished from Apollo Mission 15-17 mapping photographs. This series is also the first major effort to apply recent advances in orthophotography to lunar mapping. Presently developed maps of this series were designed to support initial lunar scientific investigations primarily employing results of Apollo Mission 15-17 data. Individual maps of this series cover 4 degrees of lunar latitude and 5 degrees of lunar longitude consisting of 1/16 of the area of a 1:1,000,000 scale Lunar Astronautical Chart (LAC) (Section 4.2.1). Their apha-numeric identification (example – LTO38B1) consists of the designator LTO for topographic orthophoto editions or LO for orthophoto editions followed by the LAC number in which they fall, followed by an A, B, C or D designator defining the pertinent LAC quadrant and a 1, 2, 3, or 4 designator defining the specific sub-quadrant actually covered. The following designation (250) identifies the sheets as being at 1:250,000 scale. The LTO editions display 100-meter contours, 50-meter supplemental contours and spot elevations in a red overprint to the base, which is lithographed in black and white. LO editions are identical except that all relief information is omitted and selenographic graticule is restricted to border ticks, presenting an umencumbered view of lunar features imaged by the photographic base. -

Earth: Atmospheric Evolution of a Habitable Planet
Earth: Atmospheric Evolution of a Habitable Planet Stephanie L. Olson1,2*, Edward W. Schwieterman1,2, Christopher T. Reinhard1,3, Timothy W. Lyons1,2 1NASA Astrobiology Institute Alternative Earth’s Team 2Department of Earth Sciences, University of California, Riverside 3School of Earth and Atmospheric Science, Georgia Institute of Technology *Correspondence: [email protected] Table of Contents 1. Introduction ............................................................................................................................ 2 2. Oxygen and biological innovation .................................................................................... 3 2.1. Oxygenic photosynthesis on the early Earth .......................................................... 4 2.2. The Great Oxidation Event ......................................................................................... 6 2.3. Oxygen during Earth’s middle chapter ..................................................................... 7 2.4. Neoproterozoic oxygen dynamics and the rise of animals .................................. 9 2.5. Continued oxygen evolution in the Phanerozoic.................................................. 11 3. Carbon dioxide, climate regulation, and enduring habitability ................................. 12 3.1. The faint young Sun paradox ................................................................................... 12 3.2. The silicate weathering thermostat ......................................................................... 12 3.3. Geological -

A Concept for the Deployment of a Large
i-SAIRAS2020-Papers (2020) 5072.pdf A CONCEPT FOR THE DEPLOYMENT OF A LARGE LUNAR CRATER RADIO TELESCOPE USING TEAMS OF TETHERED ROBOTS Virtual Conference 19–23 October 2020 Patrick McGarey1*, Saptarshi Bandyopadhyay1†, Ramin Rafizadeh1, Ashish Goel1, Manan Arya1, Issa Nesnas1, Joe Lazio1, Paul Goldsmith1, Adrian Stoica1, Marco Quadrelli1, Gregg Hallinan2 1 Jet Propulsion Laboratory, California Institute of Technology, 4800 Oak Grove Dr., Pasadena, CA, USA 91109 *[email protected], †[email protected] 2Astronomy Department, California Institute of Technology, 1200 East California Blvd, Pasadena, CA, USA 91125 ABSTRACT 1 INTRODUCTION Kilometer-scale craters on the far side of the Moon have unique potential as future locations for large ra- dio telescopes, which can observe the universe at wavelengths and frequencies (>10 m, < 30 MHz) not possible with conventional Earth or orbital-based ap- proaches. Distinct advantages of building a Lunar Crater Radio Telescope (LCRT) on the far side include i) isolation from radio noise due to the Earth’s iono- sphere, orbiting satellites, and the Sun, ii) days of un- interrupted dark/cold sky viewing during lunar night, and iii) terrain geometry naturally suited for con- structing the largest mesh antenna structure in the So- lar System. A key challenge to constructing LCRT on the Moon is related to the complexity of deploying a Figure 1: Illustration of the Lunar Crater Radio Tele- 1-km diameter antenna and hanging receiver within a scope (LCRT) concept. The green antenna reflector is lunar crater whose diameter, depth, and slope are 3-5 shown suspended by lift wires just below a suspended km, 1 km, and ~30 degrees respectively. -

User Guide to 1:250,000 Scale Lunar Maps
CORE https://ntrs.nasa.gov/search.jsp?R=19750010068Metadata, citation 2020-03-22T22:26:24+00:00Z and similar papers at core.ac.uk Provided by NASA Technical Reports Server USER GUIDE TO 1:250,000 SCALE LUNAR MAPS (NASA-CF-136753) USE? GJIDE TO l:i>,, :LC h75- lu1+3 SCALE LUNAR YAPS (Lumoalcs Feseclrch Ltu., Ottewa (Ontario) .) 24 p KC 53.25 CSCL ,33 'JIACA~S G3/31 11111 DANNY C, KINSLER Lunar Science Instltute 3303 NASA Road $1 Houston, TX 77058 Telephone: 7131488-5200 Cable Address: LUtiSI USER GUIDE TO 1: 250,000 SCALE LUNAR MAPS GENERAL In 1972 the NASA Lunar Programs Office initiated the Apollo Photographic Data Analysis Program. The principal point of this program was a detailed scientific analysis of the orbital and surface experiments data derived from Apollo missions 15, 16, and 17. One of the requirements of this program was the production of detailed photo base maps at a useable scale. NASA in conjunction with the Defense Mapping Agency (DMA) commenced a mapping program in early 1973 that would lead to the production of the necessary maps based on the need for certain areas. This paper is designed to present in outline form the neces- sary background informatiox or users to become familiar with the program. MAP FORMAT * The scale chosen for the project was 1:250,000 . The re- search being done required a scale that Principal Investigators (PI'S) using orbital photography could use, but would also serve PI'S doing surface photographic investigations. Each map sheet covers an area four degrees north/south by five degrees east/west. -

Lunar Crater Volcanic Field (Reveille and Pancake Ranges, Basin and Range Province, Nevada, USA)
Research Paper GEOSPHERE Lunar Crater volcanic field (Reveille and Pancake Ranges, Basin and Range Province, Nevada, USA) 1 2,3 4 5 4 5 1 GEOSPHERE; v. 13, no. 2 Greg A. Valentine , Joaquín A. Cortés , Elisabeth Widom , Eugene I. Smith , Christine Rasoazanamparany , Racheal Johnsen , Jason P. Briner , Andrew G. Harp1, and Brent Turrin6 doi:10.1130/GES01428.1 1Department of Geology, 126 Cooke Hall, University at Buffalo, Buffalo, New York 14260, USA 2School of Geosciences, The Grant Institute, The Kings Buildings, James Hutton Road, University of Edinburgh, Edinburgh, EH 3FE, UK 3School of Civil Engineering and Geosciences, Newcastle University, Newcastle, NE1 7RU, UK 31 figures; 3 tables; 3 supplemental files 4Department of Geology and Environmental Earth Science, Shideler Hall, Miami University, Oxford, Ohio 45056, USA 5Department of Geoscience, 4505 S. Maryland Parkway, University of Nevada Las Vegas, Las Vegas, Nevada 89154, USA CORRESPONDENCE: gav4@ buffalo .edu 6Department of Earth and Planetary Sciences, 610 Taylor Road, Rutgers University, Piscataway, New Jersey 08854-8066, USA CITATION: Valentine, G.A., Cortés, J.A., Widom, ABSTRACT some of the erupted magmas. The LCVF exhibits clustering in the form of E., Smith, E.I., Rasoazanamparany, C., Johnsen, R., Briner, J.P., Harp, A.G., and Turrin, B., 2017, overlapping and colocated monogenetic volcanoes that were separated by Lunar Crater volcanic field (Reveille and Pancake The Lunar Crater volcanic field (LCVF) in central Nevada (USA) is domi variable amounts of time to as much as several hundred thousand years, but Ranges, Basin and Range Province, Nevada, USA): nated by monogenetic mafic volcanoes spanning the late Miocene to Pleisto without sustained crustal reservoirs between the episodes. -

OSAA Boys Track & Field Championships
OSAA Boys Track & Field Championships 4A Individual State Champions Through 2006 100-METER DASH 1992 Seth Wetzel, Jesuit ............................................ 1:53.20 1978 Byron Howell, Central Catholic................................. 10.5 1993 Jon Ryan, Crook County ..................................... 1:52.44 300-METER INTERMEDIATE HURDLES 1979 Byron Howell, Central Catholic............................... 10.67 1994 Jon Ryan, Crook County ..................................... 1:54.93 1978 Rourke Lowe, Aloha .............................................. 38.01 1980 Byron Howell, Central Catholic............................... 10.64 1995 Bryan Berryhill, Crater ....................................... 1:53.95 1979 Ken Scott, Aloha .................................................. 36.10 1981 Kevin Vixie, South Eugene .................................... 10.89 1996 Bryan Berryhill, Crater ....................................... 1:56.03 1980 Jerry Abdie, Sunset ................................................ 37.7 1982 Kevin Vixie, South Eugene .................................... 10.64 1997 Rob Vermillion, Glencoe ..................................... 1:55.49 1981 Romund Howard, Madison ....................................... 37.3 1983 John Frazier, Jefferson ........................................ 10.80w 1998 Tim Meador, South Medford ............................... 1:55.21 1982 John Elston, Lebanon ............................................ 39.02 1984 Gus Envela, McKay............................................. 10.55w 1999 -

Board Certified Fellows
AMERICAN BOARD OF MEDICOLEGAL DEATH INVESTIGATORS Certificant Directory As of September 30, 2021 BOARD CERTIFIED FELLOWS Addison, Krysten Leigh (Inactive) BC2286 Allmon, James L. BC855 Travis County Medical Examiner's Office Sangamon County Coroner's Office 1213 Sabine Street 200 South 9th, Room 203 PO Box 1748 Springfield, IL 62701 Austin, TX 78767 Amini, Navid BC2281 Appleberry, Sherronda BC1721 Olmsted Medical Examiner's Office Adams and Broomfield County Office of the Coroner 200 1st Street Southwest 330 North 19th Avenue Rochester, MN 55905 Brighton, CO 80601 Applegate, MD, David T. BC1829 Archer, Meredith D. BC1036 Union County Coroner's Office Mohave County Medical Examiner 128 South Main Street 1145 Aviation Drive Unit A Marysville, OH 43040 Lake Havasu, AZ 86404 Bailey, Ted E. (Inactive) BC229 Bailey, Sanisha Renee BC1754 Gwinnett County Medical Examiner's Office Virginia Office of the Chief Medical Examiner 320 Hurricane Shoals Road, NE Central District Lawrenceville, GA 30046 400 East Jackson Street Richmond, VA 23219 Balacki, Alexander J BC1513 Banks, Elsie-Kay BC3039 Montgomery County Coroner's Office Maine Office of the Chief Medical Examiner 1430 Dekalb Street 30 Hospital Street PO Box 311 Augusta, ME 04333 Norristown, PA 19404 Bautista, Ian BC2185 Bayer, Lindsey A. BC875 New York City Office of Chief Medical Examiner District 5 and 24 Medical Examiner Office 421 East 26th Street 809 Pine Street New York, NY 10016 Leesburg, FL 34756 Beck, Shari L BC327 Beckham, Phinon Phillips BC2305 Sedgwick Co Reg. Forensic Science Center Virginia Office of the Chief Medical Examiner 1109 N. Minneapolis Northern District Wichita, KS 67214 10850 Pyramid Place, Suite 121 Manassas, VA 20110 Bednar Keefe, Gale M. -

Summary of Sexual Abuse Claims in Chapter 11 Cases of Boy Scouts of America
Summary of Sexual Abuse Claims in Chapter 11 Cases of Boy Scouts of America There are approximately 101,135sexual abuse claims filed. Of those claims, the Tort Claimants’ Committee estimates that there are approximately 83,807 unique claims if the amended and superseded and multiple claims filed on account of the same survivor are removed. The summary of sexual abuse claims below uses the set of 83,807 of claim for purposes of claims summary below.1 The Tort Claimants’ Committee has broken down the sexual abuse claims in various categories for the purpose of disclosing where and when the sexual abuse claims arose and the identity of certain of the parties that are implicated in the alleged sexual abuse. Attached hereto as Exhibit 1 is a chart that shows the sexual abuse claims broken down by the year in which they first arose. Please note that there approximately 10,500 claims did not provide a date for when the sexual abuse occurred. As a result, those claims have not been assigned a year in which the abuse first arose. Attached hereto as Exhibit 2 is a chart that shows the claims broken down by the state or jurisdiction in which they arose. Please note there are approximately 7,186 claims that did not provide a location of abuse. Those claims are reflected by YY or ZZ in the codes used to identify the applicable state or jurisdiction. Those claims have not been assigned a state or other jurisdiction. Attached hereto as Exhibit 3 is a chart that shows the claims broken down by the Local Council implicated in the sexual abuse. -

Spottiswoode-Cusack Co
SO SOUTH ESSEX AVENUE and D. L. & W R. R., ORANGE 329 WASHINGTON STREET AND ERIE R. R . --------ORANGE Spottiswoode-Cusack Co. CRUSHED STONE - - - - TELEPHONE ORANGE 3-0032 COAL TELEPHONE LUMBER TELEPHONE COAL - LUMBER - FUEL OIL KOPPERS COKE ORANGE 3-0032 ORANGE 3-3633 IRVINGTON DIRECTORY—1940 roi BARTH BAUMANN — Hugo (Tina) (Haeberle & Barth) 971 Clinton av — Mario (Lorraine) elk Newark r 46 Adams — Madeline emp Westinghouse r 55 Western pky h 40 Chapman pi Batty James W (Rose M) watchman h 357 21st — Mary A mach opr r 169 Melrose av — Hugo F Jr (Edna V) with Haeberle & Barth 971 Batz Helen J rem to Washington — Mary S wid John V h 20 Paine av Clinton av h (403) 9 Chapman pi — Raymond M rem to Washington — Norman D rem to Newark — Joseph P (Louise H) route slsman h 466 Nye av Baucski Theodore (Stephine) rem to Newark — Philip M (Marie F) metal plater h 40 Lincoln pi — Karl A (Friedal) candy mfr 37 Rich h 33 do Baudistel Arthur J elk PruInsCo r 440 Nye av — William 0 (Agnes C) contr Newark h 169 Melrose av — Katherine T wid William h 118 Laurel av — Charles E r 139 Maple av Baumbusch Albert C (Emma L) jewelrywkr h 161 Caro — Lucy r 109 Hillside ter — Eleanor elk Newark r 60 40th lina av — Milton E W (Elsie F) elk Newark h 46 Olympic ter — Emil (Helen M) macaroniwkr h 139 Maple av — Frank R metal spinner r 37 Adams — Robert H elk Millburn r 106 Florence av — Herbert (Frieda K) musician h 34 Linden av — Julius F (Louise) toolmkr h 37 Adams — William died Oct 20 1938 age 71 — Mary wid August r 34 Linden av Baumer Jacob (Helen E) bottler -

The Geology of European Coldwater Coral Carbonate Mounds the CARBONATE Project
ISSUE 30, APRIL 2010 AVAILABLE ON-LINE AT www.the-eggs.org The geology of European coldwater coral carbonate mounds the CARBONATE project Paper: ISSN 1027-6343 Online: ISSN 1607-7954 THE EGGS | ISSUE 30 | APRIL 2010 3 EGU News 4 News 11 Journal Watch 16 The geology of European coldwater coral carbonate mounds the CARBONATE project 23 Education 24 New books EDITORS Managing Editor: Kostas Kourtidis 28 Events Department of Environmental Engineering, School of Engineering Demokritus University of Thrace Vas. Sofias 12, GR-67100 Xanthi, Greece tel. +30-25410-79383, fax. +30-25410-79379 email: [email protected] 33 Job Positions Assistant Editor: Magdeline Pokar Bristol Glaciology Center, School of Geographical Sciences, University of Bristol University Road Bristol, BS8 1SS, United Kingdom tel. +44(0)117 928 8186, fax. +44(0)117 928 7878 email: [email protected] Hydrological Sciences: Guenther Bloeschl Institut fur Hydraulik, Gewasserkunde und Wasserwirtschaft Technische Universitat Wien Karlsplatz 13/223, A-1040 Wien, Austria tel. +43-1-58801-22315, fax. +43-1-58801-22399 email: [email protected] Biogeosciences: Jean-Pierre Gattuso Laboratoire d’Oceanographie de Villefranche, UMR 7093 CNRS- UPMC B. P. 28, F-06234 Villefranche-sur-mer Cedex France tel. +33-(0)493763859, fax. +33-(0)493763834 email: [email protected] Geodesy: Susanna Zerbini Department of Physics, Sector of Geophysics University of Bolo- gna, Viale Berti Pichat 8 40127 Bologna, Italy tel. +39-051-2095019, fax +39-051-2095058 e-mail: [email protected] Geodynamics: Bert L.A. Vermeersen Delft University of Technology DEOS - Fac. Aerospace Engineer- ing Astrodynamics and Satellite Systems Kluyverweg 1, NL-2629 HS Delft The Netherlands tel. -
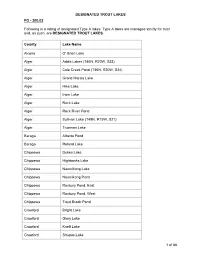
Designated Trout Lakes and Streams
DESIGNATED TROUT LAKES FO - 200.02 Following is a listing of designated Type A lakes. Type A lakes are managed strictly for trout and, as such, are DESIGNATED TROUT LAKES. County Lake Name Alcona O' Brien Lake Alger Addis Lakes (T46N, R20W, S33) Alger Cole Creek Pond (T46N, R20W, S24) Alger Grand Marais Lake Alger Hike Lake Alger Irwin Lake Alger Rock Lake Alger Rock River Pond Alger Sullivan Lake (T49N, R15W, S21) Alger Trueman Lake Baraga Alberta Pond Baraga Roland Lake Chippewa Dukes Lake Chippewa Highbanks Lake Chippewa Naomikong Lake Chippewa Naomikong Pond Chippewa Roxbury Pond, East Chippewa Roxbury Pond, West Chippewa Trout Brook Pond Crawford Bright Lake Crawford Glory Lake Crawford Kneff Lake Crawford Shupac Lake 1 of 86 DESIGNATED TROUT LAKES County Lake Name Delta Bear Lake Delta Carr Lake (T43N, R18W, S36) Delta Carr Ponds (T43N, R18W, S26) Delta Kilpecker Pond (T43N, R18W, S11) Delta Norway Lake Delta Section 1 Pond Delta Square Lake Delta Wintergreen Lake (T43N, R18W, S36) Delta Zigmaul Pond Gogebic Castle Lake Gogebic Cornelia Lake Gogebic Mishike Lake Gogebic Plymouth Lake Houghton Penegor Lake Iron Deadman’s Lk (T41N, R32W, S5 & 8) Iron Fortune Pond (T43N, R33W, S25) Iron Hannah-Webb Lake Iron Killdeer Lake Iron Madelyn Lake Iron Skyline Lake Iron Spree Lake Isabella Blanchard Pond Keweenaw Manganese Lake Keweenaw No Name Pond (T57N, R31W, S8) Luce Bennett Springs Lake Luce Brockies Pond (T46N, R11W, S1) 2 of 86 DESIGNATED TROUT LAKES County Lake Name Luce Buckies Pond (T46N, R11W, S1) Luce Dairy Lake Luce Dillingham -

Sessions Calendar
Associated Societies GSA has a long tradition of collaborating with a wide range of partners in pursuit of our mutual goals to advance the geosciences, enhance the professional growth of society members, and promote the geosciences in the service of humanity. GSA works with other organizations on many programs and services. AASP - The American Association American Geophysical American Institute American Quaternary American Rock Association for the Palynological Society of Petroleum Union (AGU) of Professional Association Mechanics Association Sciences of Limnology and Geologists (AAPG) Geologists (AIPG) (AMQUA) (ARMA) Oceanography (ASLO) American Water Asociación Geológica Association for Association of Association of Earth Association of Association of Geoscientists Resources Association Argentina (AGA) Women Geoscientists American State Science Editors Environmental & Engineering for International (AWRA) (AWG) Geologists (AASG) (AESE) Geologists (AEG) Development (AGID) Blueprint Earth (BE) The Clay Minerals Colorado Scientifi c Council on Undergraduate Cushman Foundation Environmental & European Association Society (CMS) Society (CSS) Research Geosciences (CF) Engineering Geophysical of Geoscientists & Division (CUR) Society (EEGS) Engineers (EAGE) European Geosciences Geochemical Society Geologica Belgica Geological Association Geological Society of Geological Society of Geological Society of Union (EGU) (GS) (GB) of Canada (GAC) Africa (GSAF) Australia (GSAus) China (GSC) Geological Society of Geological Society of Geologische Geoscience