A Multifaceted Overview of Apple Tree Domestication
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Page 1 the Following Pages Outline Phasing Recommendations for The
Kruckeberg Botanic Garden Master Site Plan PHASING AND IMPLEMENTATION he following pages outline phasing recommendations for the KruckebergT Botanic Garden that seem desirable to address the needs, vision, and requirements of a private garden’s evolution into the publc domain. With the transfer of this property from a private residence to a commercial public entity, new sets of codes, restrictions, and opportunities come into play. These deal with public safety, health, and well-being and ensure that equal opportunities are afforded to all. Within a limited budget, Phase 1 responds to these immediate needs by providing on-site public parking to reduce impacts to the surrounding residential community, adding much needed public restrooms, and creating a permanent and separate service access road and staff parking area. Phase 2 focuses on siting an interpretive switchback boardwalk trail that connects the upper and lower gardens in an aesthetic ADA-compliant manner. It is also envi- sioned that an ADA-compliant loop path would be routed through the lower garden. While it would be optimal to build the environmental learning center in Phase 2, it is recognized that lack of funding may require deferment to a later phase. Further development of future phases depends on many factors, most importantly securing funding and the commitment of the City, Foundation, and public to sup- port and encourage new work to proceed. In the end, this alone will determine how quickly Garden projects are completed and the Garden vision, as outlined in this report, is realized. This is a modest plan as represented by the development costs associated with each phase in 2010 dollars. -

The Vulnerability of US Apple (Malus) Genetic Resources
Genet Resour Crop Evol (2015) 62:765–794 DOI 10.1007/s10722-014-0194-2 RESEARCH ARTICLE The vulnerability of US apple (Malus) genetic resources Gayle M. Volk • C. Thomas Chao • Jay Norelli • Susan K. Brown • Gennaro Fazio • Cameron Peace • Jim McFerson • Gan-Yuan Zhong • Peter Bretting Received: 20 June 2014 / Accepted: 27 October 2014 / Published online: 13 November 2014 Ó Springer Science+Business Media Dordrecht (outside the USA) 2014 Abstract Apple (Malus 9 domestica Borkh.) is one wide range of biotic and abiotic stress resistances as of the top three US fruit crops in production and value. well as desirable productivity and fruit quality attri- Apple production has high costs for land, labor and butes. However, access to wild materials is limited and inputs, and orchards are a long-term commitment. wild Malus throughout the world is at risk of loss due Production is dominated by only a few apple scion and to human encroachment and changing climatic pat- rootstock cultivars, which increases its susceptibility terns. The USDA-ARS National Plant Germplasm to dynamic external threats. Apple crop wild relatives, System (NPGS) Malus collection, maintained by the including progenitor species Malus sieversii (Ledeb.) Plant Genetic Resources Unit in Geneva, NY, US is M. Roem., Malus orientalis Uglitzk., Malus sylvestris among the largest collections of cultivated apple and (L.) Mill., and Malus prunifolia (Willd.) Borkh., as Malus species in the world. The collection currently well as many other readily hybridized species, have a has 5004 unique accessions in the field and 1603 seed accessions representing M. 9 domestica,33Malus species, and 15 hybrid species. -

Malus Sieboldii-Based Rootstocks Mediate Apple Proliferation Resistance to Grafted Trees
Bulletin of Insectology 60 (2): 301-302, 2007 ISSN 1721-8861 Malus sieboldii-based rootstocks mediate apple proliferation resistance to grafted trees 1 2 1 Erich SEEMÜLLER , Eckard MOLL , Bernd SCHNEIDER 1Federal Biological Research Center for Agriculture and Forestry, Institute for Plant Protection in Fruit Crops, Dossenheim, Germany 2Federal Biological Research Centre for Agriculture and Forestry, Central Data Processing Group, Kleinmachnow, Germany Abstract Over a period of 24 years, three trials were carried out in which open pollinated apomictic seedlings were examined for apple pro- liferation (AP) resistance in comparison with several clonal rootstocks. The screening was performed under experimental inocula- tion and natural infection conditions. Criteria for the resistance rating were occurrence of AP symptoms, percentage of affected trees, incidence of the small fruit symptom and the effect of the disease on vigor. In all trials, similar results were obtained. Satis- factory resistance was only identified in trees grown on progenies of M. sieboldii and M. sieboldii-derived experimental root- stocks. The screening also showed that rootstocks with M. sieboldii parentage are often highly susceptible to latent apple viruses. Key words: Apple proliferation, ‘Candidatus Phytoplasma mali’, apple, resistance. Introduction clonal rootstocks of the Budagovsky series B, the Polish series P and rootstocks M 9, M 11 and M 13. The root- Apple proliferation (AP) phytoplasma disease is diffi- stocks were graft-inoculated with Golden Delicious sci- cult to control. Phytosanitary measures such as the use ons or were budded with healthy Golden Delicious and of healthy planting material and uprooting of diseased grown under natural infection conditions. Over a period trees are often unsatisfactory, because infections by in- of 24 years, three trials were carried out which where sect vectors are difficult to prevent. -

Malus) Collection and Assessment of the Crabapple Slope
University of Pennsylvania ScholarlyCommons Internship Program Reports Education and Visitor Experience 2019 Evaluation of the Crabapple (Malus) Collection and Assessment of the Crabapple Slope Micah Christensen University of Pennsylvania Follow this and additional works at: https://repository.upenn.edu/morrisarboretum_internreports Part of the Horticulture Commons Recommended Citation Christensen, Micah, "Evaluation of the Crabapple (Malus) Collection and Assessment of the Crabapple Slope" (2019). Internship Program Reports. 40. https://repository.upenn.edu/morrisarboretum_internreports/40 An independent study project report by The Charles C. Holman Endowed Rose and Flower Garden Intern (2018-2019) This paper is posted at ScholarlyCommons. https://repository.upenn.edu/morrisarboretum_internreports/40 For more information, please contact [email protected]. Evaluation of the Crabapple (Malus) Collection and Assessment of the Crabapple Slope Abstract This project began in response to space on the slope for more crabapple trees and a need to evaluate the current crabapple collection. As such, this project examined the collection as a whole with special attention to the slope. The Morris Arboretum had 48 crabapple trees as of 2018. The vast majority were planted in two locations: the slope by the rose garden and on the farm between the community garden and the executive director’s residence. The initial examination of the collection showed only two native crabapple specimens (Malus coronaria) both with a provenance of Maryland. Propagation of a tree with more local provenance was done to improve and expand the Malus collection. The four parts to this project included development and implementation of evaluation criteria, soil testing of the slope, recommendations of crabapple cultivars to fill empty spaces on the slope, and propagation of a native crabapple (Malus coronaria) with local provenance Disciplines Horticulture Comments An independent study project report by The Charles C. -

Campus Planning & Development
Planning Guidelines EEAASTSTEERRNN CCOONNNNEECCTTICICUUTT STSTAATTEE UUNNIVIVEERRSITSITYY CAMPUS PLANNING & DEVELOPMENT GUIDELINES FOR EASTERN CONNECTICUT STATE UNIVERSITY PREPARED BY NEW ENGLAND DESIGN, INC. PREPARED FOR EASTERN CONNECTICUT STATE UNIVERSITY Willimantic, Connecticut David G. Carter President Design Review Committee Nancy Tinker Committee Chair - Director of Facilities Management and Planning Tina Fu Director of Library Services Fred Gordon Student Government Association President Bob Horrocks President of the University Senate Pat Kucharski Administrative Assistant Dave Millette Electrical Shop Supervisor Michael Pernal Executive Vice President Alex Roe CSU Director of Planning & Technical Services, Ex Officio Renee Theroux-Keech Associate Director for Design and Engineering Gerald Cotter CSU Assistant Director for Project Management and Engineering PREPARED BY NEW ENGLAND DESIGN, INC. 25 Ledgebrook Drive Mansfield, CT 06250 Kevin Tubridy John Alexopoulos L.A. Karl Norton, AIA John Everett Karla Acayan Table of Contents Page Page Forward • Campus Standard Picnic Tables 11 Introduction • Campus Standard Bus Stop Shelters 11 • Campus Standard Exterior Railing and Guards 11 SITE PLANNING GUIDELINES 1 • Campus Standard Blue Phone 12 • University Lighting 12 • LANDSCAPE 1 • Campus Standard Lighting Bollards 12 • Topography and Vistas 1 • Campus Standard Solar Lighting 12 • Planting Guidelines 1 • Campus Standard Walkway and Roadway Lighting 12 • General Consideration and Observations 1 • Artwork - Sculptures, Fountains and -

Chicago Department of Transportation Roadway Plant List
CHICAGOCHICAGO DEPARTMENTDEPARTMENT OFOF TRANSPORTATIONTRANSPORTATION Richard M. Daley, Mayor Thomas H. Powers ROADWAYROADWAY PLANTPLANT LISTLIST Acting Commissioner Compiled by DIVISION OF ENGINEERING TH 7 Edition HISTORIC GARFIELD BOULEVARD CHICAGO DEPARTMENT OF TRANSPORTATION ROADWAY PLANT LIST The Chicago Department of Transportation (CDOT) Division of Engineering is pleased to present the newest edition of the City of Chicago’s Roadway Plant List, intended as a resource for landscape architects. CDOT has evaluated the plants on this list based on its years of experience maintaining medians and other roadside locations. The accompanying comments are based on the plants’ ability to withstand salt spray and other urban roadway conditions. Although no scientific trials were conducted, installation and maintenance of the plant material have been done in accordance with standard CDOT specifications. As a result, cultural conditions such as soil structure, composition and moisture are known factors. CDOT’s list highlights plant materials in order of consideration. Dark gray rows indicate plants that are not recommended. Light gray rows include plant materials that are currently under evaluation or have not yet been used. It also indicates plant selections which survive in the roadway but have attributes which negatively affect the plant’s performance in a municipal application. Although the “concerns” are independent of CDOT’s assessment of the plants’ sustenance in the roadway, CDOT expects selections to consist of plants that both survive on medians and have no additional concerns. We hope this guide proves a valuable tool for industry professionals. For more information, please contact CDOT’s Division of Engineering at (312) 744-3520. Cover Page Photo: Springtime on Historic Garfield Boulevard. -

JOURNAL of the AMERICAN HORTICULTURAL SOCIETY, INC, A.Pril 1966 AMERICAN HORTICULTURAL SOCIETY
'I'IIE A.:M:ERICAN ~GAZINE JOURNAL OF THE AMERICAN HORTICULTURAL SOCIETY, INC, A.pril 1966 AMERICAN HORTICULTURAL SOCIETY 1600 BLADENSBURG ROAD, NORTHEAST / WASHINGTON, D. c. 20002 For United Horticulture *** to accumu.late, inn-ease, and disseminate horticultuml information Editorial Committee Directors FRANCIS DE VOS, Chainnan Terms ExpiTing 1966 J. HAROLD CLARKE JOHN L . CREECH Washington f 'REDERIC P. LEE W. H . HODGE Maryland CARLTON P. LEES FREDERIC P. LEE Massachusetts RUSSELL J. SEIBERT CONRAD B. LINK Pennsylvania DONALD'VATSON FREDER ICK G. MEYER Hawaii "VII.BUR H. YOUNGMAN T erms Expi?'ing 1967 MRS. ROBERT L. EMERY, J R. Louisiana Officers A. C. HILDRETH Colorado PRESIDENT DAVID LEACH JOHN H . WALKER Pennsylvania A lexandria, Virginia CHARLES G. MEYER New Yo rk MRS. STANLEY ROWE F IRST VI CE- PRESIDENT Ohio FRED C. GALLE Terms ExjJiring 1968 Pine Mountain, Geo?-gia FRANCIS DE VOS Maryland SECOND VICE-PRESIDENT MRS. ELSA U. K NO LL TOM D. THROCKMORTON California Des Moines, Io wa VICTOR RIES Ohio STEWART D . VVI NN ACTING SECRETARY-TREASURER GRACE P. WILSON ROBERT WINTZ Bladensburg, Maryland Illinois The A merican Horticultural Magazine is the official publication of the American Horticultural Society and is issued four times a year during the quarters commencing with January, April, J!lly and October. It is devoted to the dissemination of knowledge in the science and art of growmg ornamental plants, fruits, vegetables, and related subjects. Original papers increasing the historical, varietal, and cultural knowledges of plant mate~ials of economic and aesthetic importance are welcomed and will be published as early as pOSSible. -
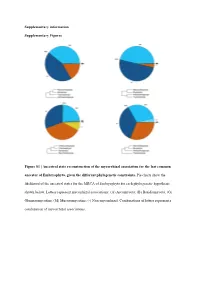
Ancestral State Reconstruction of the Mycorrhizal Association for the Last Common Ancestor of Embryophyta, Given the Different Phylogenetic Constraints
Supplementary information Supplementary Figures Figure S1 | Ancestral state reconstruction of the mycorrhizal association for the last common ancestor of Embryophyta, given the different phylogenetic constraints. Pie charts show the likelihood of the ancestral states for the MRCA of Embryophyta for each phylogenetic hypothesis shown below. Letters represent mycorrhizal associations: (A) Ascomycota; (B) Basidiomycota; (G) Glomeromycotina; (M) Mucoromycotina; (-) Non-mycorrhizal. Combinations of letters represent a combination of mycorrhizal associations. Austrocedrus chilensis Chamaecyparis obtusa Sequoiadendron giganteum Prumnopitys taxifolia Prumnopitys Prumnopitys montana Prumnopitys Prumnopitys ferruginea Prumnopitys Araucaria angustifolia Araucaria Dacrycarpus dacrydioides Dacrycarpus Taxus baccata Podocarpus oleifolius Podocarpus Afrocarpus falcatus Afrocarpus Ephedra fragilis Nymphaea alba Nymphaea Gnetum gnemon Abies alba Abies balsamea Austrobaileya scandens Austrobaileya Abies nordmanniana Thalictrum minus Thalictrum Abies homolepis Caltha palustris Caltha Abies magnifica ia repens Ranunculus Abies religiosa Ranunculus montanus Ranunculus Clematis vitalba Clematis Keteleeria davidiana Anemone patens Anemone Tsuga canadensis Vitis vinifera Vitis Tsuga mertensiana Saxifraga oppositifolia Saxifraga Larix decidua Hypericum maculatum Hypericum Larix gmelinii Phyllanthus calycinus Phyllanthus Larix kaempferi Hieronyma oblonga Hieronyma Pseudotsuga menziesii Salix reinii Salix Picea abies Salix polaris Salix Picea crassifolia Salix herbacea -
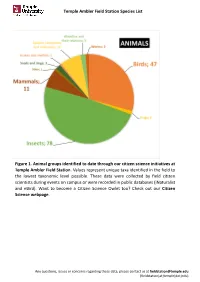
Temple Ambler Field Station Species List Figure 1. Animal Groups Identified to Date Through Our Citizen Science Initiatives at T
Temple Ambler Field Station Species List Figure 1. Animal groups identified to date through our citizen science initiatives at Temple Ambler Field Station. Values represent unique taxa identified in the field to the lowest taxonomic level possible. These data were collected by field citizen scientists during events on campus or were recorded in public databases (iNaturalist and eBird). Want to become a Citizen Science Owlet too? Check out our Citizen Science webpage. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List Figure 2. Plant diversity identified to date in the natural environments and designed gardens of the Temple Ambler Field Station and Ambler Arboretum. These values represent unique taxa identified to the lowest taxonomic level possible. Highlighted are 14 of the 116 flowering plant families present that include 524 taxonomic groups. A full list can be found in our species database. Cultivated specimens in our Greenhouse were not included here. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List database_title Temple Ambler Field Station Species List last_update 22October2020 description This database includes all species identified to their lowest taxonomic level possible in the natural environments and designed gardens on the Temple Ambler campus. These are occurrence records and each taxon is only entered once. This is an occurrence record, not an abundance record. IDs were performed by senior scientists and specialists, as well as citizen scientists visiting campus. -

Influence of Apple Stem Grooving Virus on Malus Sieboldii-Derived Apple
21st International Conference on Virus and other Graft Transmissible Diseases of Fruit Crops Influence of Apple stem grooving virus on Malus sieboldii-derived apple proliferation resistant rootstocks Liebenberg, A.; Wetzel, T.; Kappis, A.; Herdemertens M.; Krczal, G.; Jarausch, W. RLP AgroScience, AlPlanta – Institute for Plant Research, Breitenweg 71, 67435 Neustadt, Germany Abstract Apple stem grooving virus (ASGV, Capillovirus) is widely spread in apple growing regions. As it causes no symptoms on most cultivated apple varieties and rootstocks it is considered latent in Malus x domestica. In Asia, however, ASGV has been found associated with topworking disease of apple rootstocks originating from Malus sieboldii. Recently, M. sieboldii and its hybrids have gained new interest in Europe as they confer resistance to apple proliferation (AP) disease. A new breeding program aiming to develop AP-resistant rootstocks of agronomic value for modern apple culture, reported unexpected tree decline which was to be associated with ASGV. As little information is available on the variability of ASGV isolates in Germany, the complete genome of a German isolate of ASGV associated with tree decline was cloned and sequenced. Sequence comparisons with available ASGV isolates revealed two regions of high variability in the genome. The genetic variability of additional isolates from Germany and other countries were collected and the variable areas characterised. In addition ASGV was successfully maintained in micropropagated apple trees and could be transmitted by in vitro grafting to various genotypes, making it possible to study in vitro the effect of the virus and virus/phytoplama combination on M. sieboldii-derived genotypes. Keywords: Latent apple viruses, Candidatus Phytoplasma mali, micropropagation, in vitro grafting, genetic variability. -

Pip Fruits in Rose Family (Rosaceae)
Pip Fruits in Rose Family (Rosaceae) Star Performer Status In the Rose family, the pipfruit trees, (e.g., pears, apples, crab apples, and quinces) are Star Performers because of their massive flower density on the tree and the high protein content in the pollen (22%–28%). The trees or shrubs are usually deciduous with flowers opening before the leaves. Most species flower in spring with different early and late varieties. Introduction In each group there are both edible cultivars and ornamental inedible varieties. For example, the ‘flowering quince’ (Chaenomeles japonica) is grown This heritage edible pear tree (Pyrus communis)in full bloom ornamentally for the flowers, not for their fruits (although they are edible), benefits bees hugely while the closely related Cydonia oblonga and Pseudocydonia sinensis are the edible quinces. Photographer: There are both edible and ornamental cultivars of apples, pears and crab Jean- Noel Galliot apples too. Consult your local nursery. Avoid any that have double flowers or other modifications that reduce the quantity and presentation of pollen and nectar. If the spread of weeds by bird dispersal of fruits and seeds is an issue, then choose cultivars with large fruits that don’t attract birds. Domestic Apple (Malus domestica cultivars) Crab Apple (Malus species) European pear (Pyrus communis cultivars Pear flower with bee collecting pollen. Anthers in all stages of opening deliver pollen throughout the day. Photo: Jean-Noël Ornamental pear (e.g., Pyrus calleryan, P. ussuriensis, P. nivalis, P. betulaefolia) Galliot © Trees for Bees NZ. False quince (Pseudocydonia sinensis) Flowering quince (Chaenomeles species, e.g., C. japonica (Japanese flowering quince), C. -

Malus Flowering Crabapple
nysipm.cornell.edu 2019 Search for this title at the NYSIPM Publications collection: ecommons.cornell.edu/handle/1813/41246 Disease and Insect Resistant Ornamental Plants Mary Thurn, Elizabeth Lamb, and Brian Eshenaur New York State Integrated Pest Management Program, Cornell University Malus Flowering Crabapple The genus Malus includes orchard apples and crabapples. They are closely related, differing mainly in fruit size. Flowering crabapples are one of the most widely grown small ornamental trees in the Northeast and Midwest. Popular in both commercial and residential landscapes for their stunning spring flower displays, there are many selections available with a wide diversity in tree size and shape, flower color, and fruit color and persistence. Crabapples are prone to several diseases, in cluding apple scab, fire blight, powdery mildew and cedar-apple rust. The most serious fungal disease is apple scab, and susceptible cultivars may require routine preventive fungicide sprays to protect the plant. There are resistant cultivars available. However, it is important to note that there may be significant regional differences in disease resistance-a cultivar that performs well in one area may do poorly in another. There are also cultivars resistant to insect pests such as Japanese beetle, but disease resistance should be the first consideration. DISEASES Apple Scab is a disease of Malus trees caused by the fungus Venturia inaequalis. Favored by mild, wet weather, the disease causes lesions on leaves and fruit resulting in premature leaf drop and discol ored, disfigured fruit. While usually not fatal, affected trees are weakened by defoliation and lose their ornamental value. Apple Scab Reference Species/Hybrids Cultivar Resistant Susceptible Malus adstringens Hopa 3 Malus baccata Jackii 1, 3, 4, 5, 8, 11,21, 22 Malus coronaria Charlottae 5 Nieuwlandiana 5 Apple Scab Reference Species/Hybrids Cultivar Resistant Susceptible Malus coronaria var.