ORDER OF MAGNITUDE PHYSICS
RICHARD ANANTUA, JEFFREY FUNG AND JING LUAN
WEEK 2: FUNDAMENTAL INTERACTIONS, NUCLEAR AND ATOMIC PHYSICS
REVIEW OF BASICS
• Units
• Systems include SI and cgs • Dimensional analysis must confirm units on both sides of an equation match
• BUCKINGHAM’S PI THEOREM - For a physical equation involving N variables, if there are R independent dimensions, then there are N-R independent dimensionless groups, denoted Π", …, Π%&'.
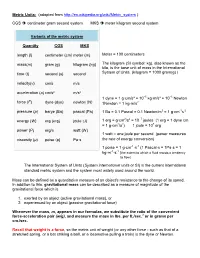
UNITS REVIEW – BASE UNITS
• Physical quantities may be expressed using several choices of units • Unit systems express physical quantities in terms of base units or combinations thereof
Quantity
Length
- SI (mks)
- Gaussian (cgs)
Centimeter (cm) Gram (g)
Imperial
- Meter (m)
- Foot (ft)
- Mass
- Kilogram (kg)
Second (s) Kelvin (K)
Pound (lb) Second (s) Farenheit (ºF)
- Time
- Second (s)
Temperature Luminous intensity Amount
Kelvin (K)*
Candela (cd) Mole (mol) Ampere (A)
Candela (cd)* Mole (mol)*
Current
* Sometimes not considered a base cgs unit
REVIEW – DERIVED UNITS
• Units may be derived from others
Quantity
Momentum Force
- SI
- cgs
- kg m s-1
- g cm s-1
Newton N=kg m s-2 Joule J=kg m2 s-2 Watt J=kg m2 s-3 dyne dyn=g cm s-2 erg=g cm2 s-2 erg/s=g cm2 s-3
Energy Power
- Pressure
- Pascal Pa=kg m-1 s-2 barye Ba=g cm-1 s-2
• Some unit systems differ in which units are considered fundamental
- Electrostatic Units
- SI (mks)
- Gaussian cgs
(cm3 g s-2)1/2 (cm3 g s-4)1/2
- Charge
- A s
- A
- Current
REVIEW – UNITS
• The cgs system for electrostatics is based on the assumptions kE=1, kM =2kE/c2
• EXERCISE: Given the Gaussian cgs unit of force is g cm s-2, what is the electrostatic unit of charge?
2
#
)/+
= g cm/ s1+ )/+
2
- ! =
- ⟹ # = ! &
[&]2
REVIEW – BUCKINGHAM’S PI THEOREM
• BUCKINGHAM’S PI THEOREM - For a physical equation involving N variables, if there are R independent dimensions, then there are N-R independent dimensionless groups, denoted Π", …, Π%&'.
• EXERCISE: What are the units of f(Π", …, Π%&')?
• We can write f(Π , …, Π )=C for dimensionless constant C
- "
- %&'
• EXERCISE: What variables are relevant for the drag force on a marble falling slowly through honey?
• Drag force Fd, Viscosity (, radius R, speed v, fluid density ρ
*+
FUNDAMENTAL PARTICLES AND INTERACTIONS
• “Atomic” concept and origins • Hierarchy of scales • Standard Model of particle physics
• Nuclear physics • Atomic physics
ICEBREAKER – PHYSICS 2 TRUTHS AND A LIE
• Make 2 true statements and 1 false statement
• 1 statement must be about yourself • 1 statement must be about your home institution • 1 statement must be about physics
HISTORY OF THE “ATOM”
• Ancient Greeks believed that matter is infinitely and continuously divisible until the advent of the Atomism
• Democritus (ca. 460B.C.-370B.C.) – Theory of Atomism:
• Matter ultimately consists of “atoms” or unchanging discrete particles
ATOMIC MODEL - HISTORY
• Democritus (ca. 460B.C.-370B.C.) – Theory of Atomic (Discrete) Matter • John Dalton (1766-1844) – Atomic Chemistry
LAW OF MULTIPLE PROPORTIONS:
If Elements A and B can form Compounds 1, 2, 3 … ,
then for a fixed mass of A, the masses of B occurring in different compounds form ratios of integers form
ATOMIC MODEL - HISTORY
• Democritus (ca. 460B.C.-370B.C.) – Theory of Atomic (Discrete) Matter • John Dalton (1766-1844) – Atomic Chemistry
- Compound
- Tin
- Tin
• EXERCISE: Tin and oxygen form the following compounds:
- oxide
- dioxide
Percentage of mass 88.1% from tin
78.7%
Percentage of mass 11.9% from oxygen
21.3%
For 100g of tin, how much oxygen is needed to make 1.) tin oxide, 2.) tin dioxide?
100g
1. ) Oxygen in tin oxide =
.119 = 13.51g
.881 100g
2.) Oxygen in tin dioxide =
.213 = 27.06g
.787
ATOMIC MODEL - HISTORY
• Democritus (ca. 460B.C.-370B.C.) – Theory of Atomic (Discrete) Matter • John Dalton (1766-1844) – Atomic Chemistry
1. Matter is comprised of small, but finite-sized atoms 2. All atoms of a given element are identical 3. Atoms cannot be destroyed 4. Compounds are formed by combining atoms in ratios of whole numbers 5. Chemical reactions are rearrangements of atoms
CHARGE CONCEPT AND HISTORY
• The Greeks also knew when certain materials (for example, amber (elektron)) were rubbed, a force could make some attract and some repel.
• William Gilbert (1544-1603) – wrote De Magnete, in which he coined
“electrius” (of amber) to describe attractive properties of charged materials
ATOMIC MODEL - HISTORY
• Democritus (ca. 460B.C.-370B.C.) – Theory of Atomic (Discrete) Matter • John Dalton (1766-1844) – Atomic Chemistry • Joseph Thomson (1856-1940) – Discovered negative “corpuscles” accelerated enough in cathode ray tubes to suggest they were 2000x lighter than H atoms
Plum Pudding Model: Electrons thought to be small negative charges immersed in the positive interior of an atom
• Thompson used electric plates and magnets in cathode ray tubes to determine e/me
ATOMIC MODEL - HISTORY
• Democritus (ca. 460B.C.-370B.C.) – Theory of Atomic (Discrete) Matter • John Dalton (1766-1844) – Atomic Chemistry • Joseph Thomson (1856-1940) – Plum Pudding Model of Atom
• Exercise: Combine fundamental constants ! , e, % and & for the electron and photon into a
- "
- "
length scale, i.e., the classical electron radius
[)] +
[-],
[3] +
[5],
67 "8
97
+
67 "8 97 :8
,
- 4
- 4
,
2
- !" =
- ⟹ !"/ = 0 1 =
- ⟹
- =
- ⟹
- = [1]
,
5
!"/, %"&,
8.99 ? 10BN ? m,/C, 1.60 ? 10EFBC, ,
- ; =
- =
- = 2.80 ⋅ 10EFN
- m
"
9.11 ⋅ 10E4Fkg 3.00 ⋅ 10Km/s
,
ATOMIC MODEL - HISTORY
• Democritus (ca. 460B.C.-370B.C.) – Theory of Atomic (Discrete) Matter • John Dalton (1766-1844) – Atomic Chemistry • Joseph Thomson (1856-1940) – Plum Pudding Model of Atom • Ernest Rutherford (1871-1937) – Fired positive !-particles at a few atoms thick gold foil sheet, and some recoiled backwards (imagine a cannonball recoiling from tissue)
• Exercise: What would be the mass of a H atom if the nucleus were filled without empty space all the way up to the electron and were the size of a marble?
+
+
- *
- 1045m
#$%&'( +
- "
- =
- "0 ≈
- + 10459kg = 10<kg
#$%&'(
10478
m
*
,-.'(-/
HIERARCHY OF SCALES
• Modern physics spans an astounding range of scales from quantum to cosmological
Observable
?e-
?
Galaxy
?
?
Universe
m
10-35
10-14
- 1
- ?
HIERARCHY OF SCALES
• Modern physics spans an astounding range of scales from quantum to cosmological
Observable e-
Galaxy
1021
Strings
10-35
Nucleus
10-14
Universe
m
10-15
1
1027
HIERARCHY OF SCALES
• Length scales can be associated with momenta via the de Broglie relation
!" = ℎ, ℎ = 6.63 ) 10,-.J ) s = 4.12 ) 10,23 eV ) s
• Rest mass can be associated with an energy scale via 4 = 869
45 = ℎ6/!
• Photon energy is related to wavelength via
Infrared Photon
UV
Radio
Photon
Planck Energy
Baryonic Acoustic Oscillation
Higgs Boson
Proton e-
Photon
Energy
1028eV
?
?
0.1TeV ?
?
0.1 eV
?
HIERARCHY OF SCALES
• Length scales can be associated with momenta via the de Broglie relation
'* = ℎ, ℎ = 6.63 / 10234J / s = 4.12 / 10289 eV / s
• Rest mass can be associated with an energy scale via ! = (%)
!" = ℎ%/'
• Photon energy is related to wavelength via
Infrared Photon
UV
Radio
Photon
Planck Energy
Baryonic Acoustic Oscillation
Higgs Boson
Proton e-
Photon
Energy
1028eV
Sound wave, not light
150 Mpc
- 0.5MeV
- 0.1 eV
0.1TeV GeV
1 neV
(1 km)
0.1 keV
(10-8 m) (10-5 m)
UNIFICATION IN PHYSICS
• Einstein unified inertial and accelerating reference frames in the general theory of relativity
• In electromagnetism, special relativity shows us electricity and magnetism are two sides of the same coin
• Other interactions in physics have been shown or predicted to be unified
ORDER OF MAGNITUDE PHYSICS
RICHARD ANANTUA, JEFFREY FUNG AND JING LUAN
WEEK 2: FUNDAMENTAL INTERACTIONS, NUCLEAR AND ATOMIC PHYSICS
REVIEW
• List these events in chronological order and name relevant scientists: Gold Foil
Experiment, Plum Pudding Model, Atomism, Law of Multiple Proportions
• Atomism (Democritus), Law of Multiple Proportions (Dalton), Plum Pudding Model (Thomson),
Gold Foil Experiment (Rutherford)
• Arrange the following in order of increasing energy needed to produce in a particle accelerator: string, electron, proton.
• Electron, proton, string
• Two Truths and a Lie
• ____ hates physics • Daulande doesn’t have a ____
REVIEW
• List these events in chronological order and name relevant scientists: Gold Foil
Experiment, Plum Pudding Model, Atomism, Law of Multiple Proportions
• Atomism (Democritus), Law of Multiple Proportions (Dalton), Plum Pudding Model (Thomson),
Gold Foil Experiment (Rutherford)
• Arrange the following in order of increasing energy needed to produce in a particle accelerator: string, electron, proton.
• Electron, proton, string
• Two Truths and a Lie
• Arthur hates physics • Daulande doesn’t have a ____
REVIEW
• List these events in chronological order and name relevant scientists: Gold Foil
Experiment, Plum Pudding Model, Atomism, Law of Multiple Proportions
• Atomism (Democritus), Law of Multiple Proportions (Dalton), Plum Pudding Model (Thomson),
Gold Foil Experiment (Rutherford)
• Arrange the following in order of increasing energy needed to produce in a particle accelerator: string, electron, proton.
• Electron, proton, string
• Two Truths and a Lie
• Arthur hates physics • Daulande doesn’t have a goldfish
STANDARD MODEL OF PARTICLE PHYSICS
• The Standard Model is currently comprised of
• (Integer+1/2)-spin fermions and integer-spin bosons • 3 generations of fermions • Electromagnetic, weak and strong gauge bosons
• Fermions (but not bosons) obey the
• PAULI EXCLUSION PRINCIPLE – no two half-spin particles can occupy the same quantum state
QUARKS MULTIPLETS
• Hadrons are combinations of quarks “glued” together by gluons via strong interaction
- "
- #
• Mesons, such as ! and ! , are quark doublets • Baryons, such as p and n, are quark triples
Q=? s=?
Q=? s=?
Q=? s=?
Q=? s=?
• Quarks may exist in combinations of 5’s known as pentaquarks • Leptons, such as electrons and muons, have no quark substructure and do not partake in the strong interaction
QUARKS MULTIPLETS
• Hadrons are combinations of quarks “glued” together by gluons via strong interaction
- "
- #
• Mesons, such as ! and ! , are quark doublets • Baryons, such as p and n, are quark triples
- Q=1
- Q=-1
s=0
Q=1 s=1/2
Q=0 s=1/2
• Quarks may exist in combinations of 5’s known ass=p0entaquarks • Leptons, such as electrons and muons, have no quark substructure and do not partake in the strong interaction
SPONTANEOUS SYMMETRY BREAKING
• Feynman devised diagrams modeling forces (electromagnetic, weak and strong) as interactions mediated by exchange particles
• Photons carry the electromagnetic force • W and Z bosons carry the weak force
t
Richard Feynman 1918-1988
• Gluons carry the strong force • At high energy, symmetry emerges among
E
force-carrying Goldstone bosons
• Many low energy states available to break
symmetry
UNIFICATION IN PHYSICS
• Einstein unified inertial and accelerating reference frames in the general theory of relativity
• In electromagnetism, special relativity shows us electricity and magnetism are two sides of the same coin
• At the electroweak energy scale (246 GeV), the electromagnetic force is
indistinguishable from the weak force
• At the strong-electroweak scale, the electroweak force is predicted by grand unified
theory to be indistinguishable from the strong force. Proton decay detectors such as super Kamiokande have yet to confirm this prediction