Nickel Silver
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHEMICAL POLISHING of NICKEL - SILVER ORNAMENTS MRS MALATHY PUSHPA VANAM, S JOHN and B a SHENOI Central Electrochemical Research Institute, Karaikudi - 623 006
Bulletm of ledr roc hem is try 1 (4) July-August 1985, pp. 381-383 CHEMICAL POLISHING OF NICKEL - SILVER ORNAMENTS MRS MALATHY PUSHPA VANAM, S JOHN AND B A SHENOI Central Electrochemical Research Institute, Karaikudi - 623 006 ABSTRACT A bath composition has been selected for descaling and chemical polishmg of n~ckel-s~lver artcles and the effect of each constituent of the bath on its polishing abllity has been studied. Key words: Chemical polishing, Ni-Ag alloy INTRODUCTION The polished specimens were immediately washed and rinsed. A thin film of the white metal hydroxides still adhering he alloys usually called nickel - silver, white metal or to the surface was removed in order to get a clear brilliant German silver are copper --nickel- zinc alloys or nickel T surface and also to obtain adherent silver coating in the subse- brasses [I]. The three most common Cu - Ni - Zn alloys quent step. Wasing in water alone or brushing did not solve contain 72%, 65% and 55 % copper, 18% nickel and the this problem. Treatment in any mineral acid, however dilute it remainder zinc. They are used in spring applications, gift and be, reduced the lustre of the initial surface. Hence, treatmen' tableware-usually silver plated, in musical and dental in a dilute solution of a complexing agent like EDTA, citric instruments, slide fastenen and as a base metal for moderate acid, tartaric acid etc. capable of complexing nickel and prized jewellery with or without plated coatings [2]. copper salts were tried. The articles after thorough washing Because of the lack of ductility at rqom temperature, this were rinsed and transferred to the silver plating solution. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

Metals and Metal Products Tariff Schedules of the United States
251 SCHEDULE 6. - METALS AND METAL PRODUCTS TARIFF SCHEDULES OF THE UNITED STATES SCHEDULE 6. - METALS AND METAL PRODUCTS 252 Part 1 - Metal-Bearing Ores and Other Metal-Bearing Schedule 6 headnotes: Materials 1, This schedule does not cover — Part 2 Metals, Their Alloys, and Their Basic Shapes and Forms (II chemical elements (except thorium and uranium) and isotopes which are usefully radioactive (see A. Precious Metals part I3B of schedule 4); B. Iron or Steel (II) the alkali metals. I.e., cesium, lithium, potas C. Copper sium, rubidium, and sodium (see part 2A of sched D. Aluminum ule 4); or E. Nickel (lii) certain articles and parts thereof, of metal, F. Tin provided for in schedule 7 and elsewhere. G. Lead 2. For the purposes of the tariff schedules, unless the H. Zinc context requires otherwise — J. Beryllium, Columbium, Germanium, Hafnium, (a) the term "precious metal" embraces gold, silver, Indium, Magnesium, Molybdenum, Rhenium, platinum and other metals of the platinum group (iridium, Tantalum, Titanium, Tungsten, Uranium, osmium, palladium, rhodium, and ruthenium), and precious- and Zirconium metaI a Iloys; K, Other Base Metals (b) the term "base metal" embraces aluminum, antimony, arsenic, barium, beryllium, bismuth, boron, cadmium, calcium, chromium, cobalt, columbium, copper, gallium, germanium, Part 3 Metal Products hafnium, indium, iron, lead, magnesium, manganese, mercury, A. Metallic Containers molybdenum, nickel, rhenium, the rare-earth metals (Including B. Wire Cordage; Wire Screen, Netting and scandium and yttrium), selenium, silicon, strontium, tantalum, Fencing; Bale Ties tellurium, thallium, thorium, tin, titanium, tungsten, urani C. Metal Leaf and FoU; Metallics um, vanadium, zinc, and zirconium, and base-metal alloys; D, Nails, Screws, Bolts, and Other Fasteners; (c) the term "meta I" embraces precious metals, base Locks, Builders' Hardware; Furniture, metals, and their alloys; and Luggage, and Saddlery Hardware (d) in determining which of two or more equally specific provisions for articles "of iron or steel", "of copper", E. -

The Periodic Table of Elements
The Periodic Table of Elements 1 2 6 Atomic Number = Number of Protons = Number of Electrons HYDROGENH HELIUMHe 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 Atomic Weight 11 12 14 16 19 20 11 12 13 14 15 16 17 18 SODIUMNa MAGNESIUMMg ALUMINUMAl SILICONSi PHOSPHORUSP SULFURS CHLORINECl ARGONAr 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 POTASSIUMK CALCIUMCa SCANDIUMSc TITANIUMTi VANADIUMV CHROMIUMCr MANGANESEMn FeIRON COBALTCo NICKELNi CuCOPPER ZnZINC GALLIUMGa GERMANIUMGe ARSENICAs SELENIUMSe BROMINEBr KRYPTONKr 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 RUBIDIUMRb STRONTIUMSr YTTRIUMY ZIRCONIUMZr NIOBIUMNb MOLYBDENUMMo TECHNETIUMTc RUTHENIUMRu RHODIUMRh PALLADIUMPd AgSILVER CADMIUMCd INDIUMIn SnTIN ANTIMONYSb TELLURIUMTe IODINEI XeXENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 CESIUMCs BARIUMBa HAFNIUMHf TANTALUMTa TUNGSTENW RHENIUMRe OSMIUMOs IRIDIUMIr PLATINUMPt AuGOLD MERCURYHg THALLIUMTl PbLEAD BISMUTHBi POLONIUMPo ASTATINEAt RnRADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 FRANCIUMFr RADIUMRa RUTHERFORDIUMRf DUBNIUMDb SEABORGIUMSg BOHRIUMBh HASSIUMHs MEITNERIUMMt DARMSTADTIUMDs ROENTGENIUMRg COPERNICIUMCn NIHONIUMNh -

The Nickel Silvers
Copper Development Association The Nickel Silvers Design Data and Applications 1965 Please note this publication is provided as an archive copy. The information given may therefore not be current. The Nickel Silvers Design Data and Applications 1965 Copper Development Association Copper Development Association is a non-trading organisation sponsored by the copper producers and fabricators to encourage the use of copper and copper alloys and to promote their correct and efficient application. Its services, which include the provision of technical advice and information, are available to those interested in the utilisation of copper in all its aspects. The Association also provides a link between research and user industries and maintains close contact with other copper development associations throughout the world. Website: www.copperinfo.co.uk Email: [email protected] Copyright: All information in this document is the copyright of Copper Development Association Disclaimer: Whilst this document has been prepared with care, Copper Development Association can give no warranty regarding the contents and shall not be liable for any direct, indirect or consequential loss arising out of its use Contents Contents ...............................................................................................................................................................1 Introduction .........................................................................................................................................................2 -

Copper Alloys
THE COPPER ADVANTAGE A Guide to Working With Copper and Copper Alloys www.antimicrobialcopper.com CONTENTS I. Introduction ............................. 3 PREFACE Conductivity .....................................4 Strength ..........................................4 The information in this guide includes an overview of the well- Formability ......................................4 known physical, mechanical and chemical properties of copper, Joining ...........................................4 as well as more recent scientific findings that show copper has Corrosion ........................................4 an intrinsic antimicrobial property. Working and finishing Copper is Antimicrobial ....................... 4 techniques, alloy families, coloration and other attributes are addressed, illustrating that copper and its alloys are so Color ..............................................5 adaptable that they can be used in a multitude of applications Copper Alloy Families .......................... 5 in almost every industry, from door handles to electrical circuitry to heat exchangers. II. Physical Properties ..................... 8 Copper’s malleability, machinability and conductivity have Properties ....................................... 8 made it a longtime favorite metal of manufacturers and Electrical & Thermal Conductivity ........... 8 engineers, but it is its antimicrobial property that will extend that popularity into the future. This guide describes that property and illustrates how it can benefit everything from III. Mechanical -

Special-Purpose Nickel Alloys
© 2000 ASM International. All Rights Reserved. www.asminternational.org ASM Specialty Handbook: Nickel, Cobalt, and Their Alloys (#06178G) Special-Purpose Nickel Alloys NICKEL-BASE ALLOYS have a number of meet special needs. The grades considered in ganese, and copper, a 0.005% limit on iron, and unique properties, or combinations of proper- this section include the following: a 0.02% limit on carbon. This high purity re- ties, that allow them to be used in a variety of sults in lower coefficient of expansion, electri- specialized applications. For example, the high • Nickel 200 (99.6% Ni, 0.04% C) cal resistivity, Curie temperature, and greater resistivity (resistance to flow of electricity) and • Nickel 201 (99.6% Ni, 0.02% C maximum) ductility than those of other grades of nickel heat resistance of nickel-chromium alloys lead • Nickel 205 (99.6% Ni, 0.04% C, 0.04% Mg) and makes Nickel 270 especially useful for to their use as electric resistance heating ele- • Nickel 233 (see composition in table that fol- some electronics applications such as compo- ments. The soft magnetic properties of lows) nents of hydrogen thyratrons and as a substrate nickel-iron alloys are employed in electronic • Nickel 270 (99.97% Ni) for precious metal cladding. devices and for electromagnetic shielding of computers and communication equipment. Iron- Composition limits and property data on sev- eral of these grades can be found in the article nickel alloys have low expansion characteris- Resistance Heating Alloys tics as a result of a balance between thermal ex- “Wrought Corrosion-Resistant Nickels and pansion and magnetostrictive changes with Nickel Alloys” in this Handbook. -

Properties of Gold-Nickel Alloy Brazed Joints in High Temperature Materials
Properties of Gold-Nickel Alloy Brazed Joints in High Temperature Materials Professor Jakob Colbus University of Saarland, Saarbrücken, West Germany and Karl Friedrich Zimmermann Degussa, Wolfgang, West Germany The usefulness of gold-nickel alloys as brazing media in the aero- engine, electronic, nuclear power and spacecraft industries is illustrated by data on the corrosion and oxidation resistance of joints made with these alloys in various heat-resistant materials and on their resistance to fracture under the influence of static, dynamic and impact loads in tests at subzero, normal and elevated temperatures. Gold solders have been in use for many years, Brazing temperature in the range 900 to 1000°C. especially in the jewellery trade and in dentistry. Ability to make joints that retain their strength The solders used in the fabrication of jewellery and ductility at elevated temperatures. contain silver, copper, cadmium, and zinc, which are Good resistance to oxidation and corrosion. added to ensure that the colour and the gold content All these requirements can be satisfied by several of a given solder matches the corresponding charac- alloys in the gold-nickel system, whose constitutional teristics of the soldered article. The solders used in diagram is shown in Figure 1. The 18 per cent dental practice often contain other precious metals nickel-gold alloy is the most suitable one to serve as a such as iridium, palladium, and platinum introduced brazing medium. Its melting point of 950°C is the to increase their corrosion resistance, the most important property of solders of this kind. In both these applications the soldering is done in air with WEIGHT PERCENT NICKEL the aid of a gas torch and a suitable flux. -

Mechanical Properties of Electroformed Nickel Cobalt Alloys
Mechanical Properties of Electroformed Nickel Cobalt Alloys B. Stein, P. Jaeger and C. Przybyla, NiCoForm, Inc., Rochester, New York, USA Abstract Hardness was measured on ½" - thick stainless steel blocks plated with at least 0.02" of Ni-Co on a Mechanical properties of nickel-cobalt alloys (0-10% Rockwell superficial hardness tester (Wilson Co) electroformed with low internal stress in Instruments Mod. 3 JS) with a 15kg load. The sulfamate-based solutions were investigated in as average of three readings was taken for each data point deposited and heat treated conditions. It was in tensile and hardness tests. demonstrated that alloy properties can be modified by selecting deposition conditions and alloy composition. Both foil samples and hardness test blocks were Recommendations on alloy selection are made based electroformed at 1-3 A/Dm2 in two proprietary Ni-Co on application requirements. alloy electroforming chemistries, one designed for enhanced deposit hardness, the other - for high tensile The higher strength and hardness of nickel-cobalt alloy strength. Deposit internal stress was measured directly electrodeposits compared to nickel stimulates their in the plating tanks as described elsewhere [1], while increasing use in electroforming and as engineering tensile and hardness samples were being coatings. Many advanced electroformed products such electroformed. Tensile and hardness tests were as bellows, contacts, optical and nanofluidic molds can performed on samples in the as plated and heat treated only be produced consistently if the deposits’ (2 hours at the set temperature) conditions. properties are well understood and properly controlled Representative foil samples were chemically analyzed by the process engineer. -

Handbook : Brass, Bronze, Copper, Nickel Silver
•ui#:.r fc ::* £ \ ' TONKt* * i i "t ' 1 .'.'. i HANDBOOK tt BRASS • BRONZE • COPPER NICKEL SILVER I fc Anacon dA from mine to consumer • IftUitilHlli'l J July 1, 1935 3 h * THE AMERICAN BRASS COMPANY 3 * *.. Copyright, 1935 The American Brass Company Printed in U. S. A. C THE AMERICAN BRASS COMPANY General Offices WATERBURY, CONNECTICUT, U.S.A. Manufacturing Plants ANSONIA, CONN. TORRINGTON, CONN. WATERBURY, CONN. BUFFALO, N. Y. DETROIT, MICH. KENOSHA, WIS. Offices and Agencies BOSTON, MASS. 140 Federal Street PROVIDENCE, R. I. 131 Dorrance Strei 1 NEW YORK, N. Y. 25 Broadway SYRACUSE, N. Y. 207 East Genesee Street Place NEWARK, N. J. 20 Branford WASHINGTON, D. C. 1511 K Street, N. W. PHILADELPHIA, PA. 117 South Seventeenth Strei t PITTSBURGH, PA. 535 Smithfield Street CLEVELAND. OHIO 925 Euclid Avenue DAYTON, OHIO 52 North Main Street CINCINNATI, OHIO 101 West Fourth Street CHICAGO, ILL. 1326 West Washington Boulevard ST. LOUIS, MO. 408 Pine Street ATLANTA. GA. 10 Forsyth Street HOUSTON, TEXAS 609 Fannin Street DENVER, COLO. 818 Seventeenth Street LOS ANGELES, CALIF. 411 Weai Fifth Street SAN FRANCISCO, CALIF. 235 Montgomery Street SEATTLE, WASH. 1358 Fourth Avenue THE AMERICAN BRASS COMPANY OF ILLINOIS 1326 West Washington Boulevard, Chicago, 111. In Canada ANACONDA AMERICAN BRASS LIMITED Main Office and Mill NEW TORONTO, ONTARIO Montreal Agency: 1010 St. Catherine Street, W. * 3 CABLE ADDRESSES "AMBRAC" Waterbury, Conn. "AMBRAC" 25 Broadway, New York Codes Used All Standard Cable and Telegraph THE AMERICAN BRASS COMPANY - Tl ANACONDA -
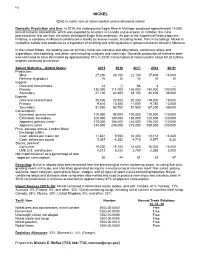
Nickel Data Sheet
112 NICKEL (Data in metric tons of nickel content unless otherwise noted) Domestic Production and Use: In 2019, the underground Eagle Mine in Michigan produced approximately 14,000 tons of nickel in concentrate, which was exported to smelters in Canada and overseas. In October, the mine processed the first ore from the newly developed Eagle East extension. As part of the Superfund Redevelopment Initiative, a company in Missouri constructed a facility to recover metals, including nickel, from mine tailings. Nickel in crystalline sulfate was produced as a byproduct of smelting and refining platinum-group-metal ores mined in Montana. In the United States, the leading uses for primary nickel are stainless and alloy steels, nonferrous alloys and superalloys, electroplating, and other uses including catalysts and chemicals. Domestic production of stainless steel was estimated to have decreased by approximately 10% in 2019. Consumption of nickel used in alloys for jet turbine engines continued to increase. Salient Statistics—United States: 2015 2016 2017 2018 2019e Production: Mine 27,200 24,100 22,100 17,600 14,000 Refinery, byproduct W W W W W Imports: Ores and concentrates 24 (1) 64 3 — Primary 130,000 111,000 150,000 144,000 120,000 Secondary 27,100 32,300 38,100 45,100 38,000 Exports: Ores and concentrates 25,400 22,400 20,000 219,000 19,000 Primary 9,610 10,300 11,000 9,780 13,000 Secondary 51,900 63,700 51,500 67,200 49,000 Consumption: Estimated, primary metal 110,000 98,000 100,000 110,000 110,000 Estimated, secondary 120,000 130,000 130,000 120,000 120,000 Apparent, primary metal3 118,000 104,000 140,000 136,000 110,000 Apparent, total4 234,000 235,000 273,000 259,000 230,000 Price, average annual, London Metal Exchange (LME): Cash, dollars per metric ton 11,831 9,594 10,403 13,114 14,000 Cash, dollars per pound 5.367 4.352 4.719 5.977 6.30 Stocks, yearend: Consumer 19,200 15,100 14,600 16,300 16,000 LME U.S. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table.