A Review of Gastrolith Function with Implications for Fossil Vertebrates and a Revised Classification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mesozoic—Dinos!
MESOZOIC—DINOS! VOLUME 9, ISSUE 8, APRIL 2020 THIS MONTH DINOSAURS! • Dinosaurs ○ What is a Dinosaur? page 2 DINOSAURS! When people think paleontology, ○ Bird / Lizard Hip? page 5 they think of scientists ○ Size Activity 1 page 10 working in the hot sun of ○ Size Activity 2 page 13 Colorado National ○ Size Activity 3 page 43 Monument or the Badlands ○ Diet page 46 of South Dakota and ○ Trackways page 53 Wyoming finding enormous, ○ Colorado Fossils and fierce, and long-gone Dinosaurs page 66 dinosaurs. POWER WORDS Dinosaurs safely evoke • articulated: fossil terror. Better than any bones arranged in scary movie, these were Articulated skeleton of the Tyrannosaurus rex proper order actually living breathing • endothermic: an beasts! from the American Museum of Natural History organism produces body heat through What was the biggest dinosaur? be reviewing the information metabolism What was the smallest about dinosaurs, but there is an • metabolism: chemical dinosaur? What color were interview with him at the end of processes that occur they? Did they live in herds? this issue. Meeting him, you will within a living organism What can their skeletons tell us? know instantly that he loves his in order to maintain life What evidence is there so that job! It doesn’t matter if you we can understand more about become an electrician, auto CAREER CONNECTION how these animals lived. Are mechanic, dancer, computer • Meet Dr. Holtz, any still alive today? programmer, author, or Dinosaur paleontologist, I truly hope that Paleontologist! page 73 To help us really understand you have tremendous job more about dinosaurs, we have satisfaction, like Dr. -

Onetouch 4.0 Scanned Documents
/ Chapter 2 THE FOSSIL RECORD OF BIRDS Storrs L. Olson Department of Vertebrate Zoology National Museum of Natural History Smithsonian Institution Washington, DC. I. Introduction 80 II. Archaeopteryx 85 III. Early Cretaceous Birds 87 IV. Hesperornithiformes 89 V. Ichthyornithiformes 91 VI. Other Mesozojc Birds 92 VII. Paleognathous Birds 96 A. The Problem of the Origins of Paleognathous Birds 96 B. The Fossil Record of Paleognathous Birds 104 VIII. The "Basal" Land Bird Assemblage 107 A. Opisthocomidae 109 B. Musophagidae 109 C. Cuculidae HO D. Falconidae HI E. Sagittariidae 112 F. Accipitridae 112 G. Pandionidae 114 H. Galliformes 114 1. Family Incertae Sedis Turnicidae 119 J. Columbiformes 119 K. Psittaciforines 120 L. Family Incertae Sedis Zygodactylidae 121 IX. The "Higher" Land Bird Assemblage 122 A. Coliiformes 124 B. Coraciiformes (Including Trogonidae and Galbulae) 124 C. Strigiformes 129 D. Caprimulgiformes 132 E. Apodiformes 134 F. Family Incertae Sedis Trochilidae 135 G. Order Incertae Sedis Bucerotiformes (Including Upupae) 136 H. Piciformes 138 I. Passeriformes 139 X. The Water Bird Assemblage 141 A. Gruiformes 142 B. Family Incertae Sedis Ardeidae 165 79 Avian Biology, Vol. Vlll ISBN 0-12-249408-3 80 STORES L. OLSON C. Family Incertae Sedis Podicipedidae 168 D. Charadriiformes 169 E. Anseriformes 186 F. Ciconiiformes 188 G. Pelecaniformes 192 H. Procellariiformes 208 I. Gaviiformes 212 J. Sphenisciformes 217 XI. Conclusion 217 References 218 I. Introduction Avian paleontology has long been a poor stepsister to its mammalian counterpart, a fact that may be attributed in some measure to an insufRcien- cy of qualified workers and to the absence in birds of heterodont teeth, on which the greater proportion of the fossil record of mammals is founded. -
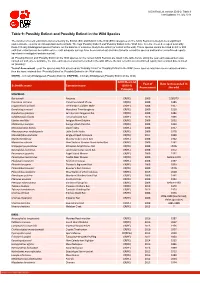
Table 9: Possibly Extinct and Possibly Extinct in the Wild Species
IUCN Red List version 2019-2: Table 9 Last Updated: 18 July 2019 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". Year of Assessment - year the species was first assessed as 'Possibly Extinct' or 'Possibly Extinct in the Wild'; some species may have been reassessed since then but have retained their 'Possibly Extinct' or 'Possibly Extinct in the Wild' status. CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild), IUCN Red List Year of Date last recorded in -

Evolution of Galliformes and Their Presence in the Carpathian Basin
Ornis Hungarica 2019. 27(2): 142–174. DOI: 10.2478/orhu-2019-0021 Evolution of Galliformes and their presence in the Carpathian Basin Jenő (Eugen) KESSLER Received: April 23, 2019 – Revised: May 13, 2019 – Accepted: May 15, 2019 Kessler, J. (E.) 2019. Evolution of Galliformes and their presence in the Carpathian Basin. – Or- nis Hungarica 27(2): 142–174. DOI: 10.2478/orhu-2019-0021 Abstract Due to the number of specimen, their size and weaker flight capabilities they are one of the favorite preys of furred and feathered carnivores. Due to this factor quite a number of skeletal fragments remained and fossilized over millions of years, especially in caves. Their presence in Europe can be traced back to the Eocene, but the majority of finds come from the Neogene and the Quaternary. In the Car- pathian Basin they are known since the beginning of the Neogene. The text is complemented with the bibliogra- phy concerning the fossilized material, one figure and six table. Keywords: Europe, Carpathian Basin, evolution, Galliformes, grouses Összefoglalás Egyedszámuk, méretük, életmódjuk, és gyengébb repülési képességük következtében kedvenc prédaállataik a tollas és szőrmés ragadozóknak. Az előbbieknek köszönhetően így elég sok vázrészük fennma- radt és fosszilizálódhatott az évmilliók folyamán, főleg a barlangi lelőhelyeken. Európai jelenlétüket már az eo- céntól követni lehet, de a leletek többsége a neogénből és a kvarterből származik. A Kárpát-medencéből a neo- gén elejétől ismertek. A szöveget kiegészíti a fosszilis anyagot felölelő irodalomjegyzék, egy ábra és hat táblázat. Kulcsszavak: Európa, Kárpát-medence, evolúció, tyúkfélék, fajdok Department of Paleontology, Eötvös Loránd University, 1117 Budapest, Pázmány Péter sétány 1/c, Hungary, e-mail: [email protected] Introduction Their dimensions vary (sexual dimorphism is a usual characteristic), they are herbivores or omnivores. -

Gastroliths in an Ornithopod Dinosaur
Brief report Acta Palaeontologica Polonica 53 (2): 351–355, 2008 Gastroliths in an ornithopod dinosaur IGNACIO A. CERDA Gastroliths (stomach stones) are known from many extant Institutional abbreviations.—MCSPv, Vertebrate paleontology and extinct vertebrates, including dinosaurs. Reported here collection of the Museo de Cinco Saltos, Río Negro Province, is the first unambiguous record of gastroliths in an ornitho− Argentina; MUCPv, Vertebrate paleontology collection of the pod dinosaur. Clusters of small stones found in the abdomi− Museo de la Universidad Nacional del Comahue, Neuquén nal region of three articulated skeletons of Gasparinisaura Province, Argentina. cincosaltensis were identified as gastroliths on the basis of taphonomic and sedimentologic evidence. The large number Material and geologic setting of stones found in each individual, their size, and the fact that Gasparinisaura cincosaltensis was herbivorous, all sug− Three specimens of Gasparinisaura cincosaltensis, MUCPv 213, gest that they were ingested as a result of lithophagy rather MCSPv 111, and MCSPv 112, were collected near the city of than accidental swallowing. Cinco Saltos (Río Negro Province, Patagonia, Argentina) (Fig. 1), in mudstones and sandstones of the early Campanian Anacleto Introduction Formation, in the uppermost portion of the Neuquén Group (Ramos 1981; Dingus et al. 2000). MUCPv 213 (Fig. 2A) consists Gastroliths or geo−gastroliths sensu Wings (2007) are known in of a partial skeleton that includes cranial and postcranial elements many taxa of extant and fossil vertebrates (Whittle and Everhart (see Salgado et al. 1997 for a detailed anatomical description). A 2000). Gastroliths have been occasionally reported in non−avian portion of the preserved elements (both incomplete humeri articu− dinosaurs (Wings 2004) but only few cases can withstand rigorous lated with both radii and ulnae, several posterior dorsal ribs from testing. -

Late Jurassic Dinosaurs on the Move, Gastroliths and Long-Distance Migration" (2019)
Augustana College Augustana Digital Commons Geography: Student Scholarship & Creative Works Geography Winter 12-8-2019 Late Jurassic Dinosaurs on the Move, Gastroliths and Long- Distance Migration Josh Malone Augustana College, Rock Island Illinois Follow this and additional works at: https://digitalcommons.augustana.edu/geogstudent Part of the Geology Commons, Physical and Environmental Geography Commons, Sedimentology Commons, and the Spatial Science Commons Augustana Digital Commons Citation Malone, Josh. "Late Jurassic Dinosaurs on the Move, Gastroliths and Long-Distance Migration" (2019). Geography: Student Scholarship & Creative Works. https://digitalcommons.augustana.edu/geogstudent/8 This Student Paper is brought to you for free and open access by the Geography at Augustana Digital Commons. It has been accepted for inclusion in Geography: Student Scholarship & Creative Works by an authorized administrator of Augustana Digital Commons. For more information, please contact [email protected]. LATE JURASSIC DINOSAURS ON THE MOVE, GASTROLITHS AND LONG- DISTANCE MIGRATION a senior thesis written by Joshua Malone in partial fulfillment of the graduation requirements for the major in Geography Augustana College Rock Island, Illinois 61201 1 Table of Contents 1. Abstract ................................................................................................................................................ 4 2. Introduction ........................................................................................................................................ -

Bending the Curve of Global Freshwater Biodiversity Loss
Forum Bending the Curve of Global Freshwater Biodiversity Loss: Downloaded from https://academic.oup.com/bioscience/advance-article-abstract/doi/10.1093/biosci/biaa002/5732594 by guest on 19 February 2020 An Emergency Recovery Plan DAVID TICKNER, JEFFREY J. OPPERMAN, ROBIN ABELL, MIKE ACREMAN, ANGELA H. ARTHINGTON, STUART E. BUNN, STEVEN J. COOKE, JAMES DALTON, WILL DARWALL, GAVIN EDWARDS, IAN HARRISON, KATHY HUGHES, TIM JONES, DAVID LECLÈRE, ABIGAIL J. LYNCH, PHILIP LEONARD, MICHAEL E. MCCLAIN, DEAN MURUVEN, JULIAN D. OLDEN, STEVE J. ORMEROD, JAMES ROBINSON, REBECCA E. THARME, MICHELE THIEME, KLEMENT TOCKNER, MARK WRIGHT, AND LUCY YOUNG Despite their limited spatial extent, freshwater ecosystems host remarkable biodiversity, including one-third of all vertebrate species. This biodiversity is declining dramatically: Globally, wetlands are vanishing three times faster than forests, and freshwater vertebrate populations have fallen more than twice as steeply as terrestrial or marine populations. Threats to freshwater biodiversity are well documented but coordinated action to reverse the decline is lacking. We present an Emergency Recovery Plan to bend the curve of freshwater biodiversity loss. Priority actions include accelerating implementation of environmental flows; improving water quality; protecting and restoring critical habitats; managing the exploitation of freshwater ecosystem resources, especially species and riverine aggregates; preventing and controlling nonnative species invasions; and safeguarding and restoring river connectivity. -

A Simulated Bird Gastric Mill and Its Implications for Fossil Gastrolith Authenticity
Fossil Record 12 (1) 2009, 91–97 / DOI 10.1002/mmng.200800013 A simulated bird gastric mill and its implications for fossil gastrolith authenticity Oliver Wings Steinmann-Institut fr Geologie, Mineralogie und Palontologie, Universitt Bonn, Nussallee 8, 53115 Bonn, Germany. E-mail: [email protected] Current address: Museum fr Naturkunde Berlin, Invalidenstraße 43, 10115 Berlin, Germany. Abstract Received 12 June 2008 A rock tumbler, stones, water, plant material, hydrochloric acid, and pepsin were used Accepted 15 August 2008 to simulate a bird gizzard in order to study abrasion rate and influence of stomach Published 20 February 2009 juices and foodstuff on gastrolith surface development. The experiment lasted for six months. Each week, the “stomach” was supplied with fresh grass and stomach juices. After the end of the experiment, the set of stones had a combined weight loss of 22.4%, with softer rock types showing higher abrasion rates. The combination of sto- mach juices and silica phytoliths within the grass had no visible effect on stone surface development: polish or pitting did not occur. A second experiment combined only peb- bles with water in the tumbler. Results indicate that rock abrasion is mainly caused by contacts between moving stones. A comparison with authentic ostrich gastroliths Key Words showed that abrasion in the artificial stomach must have been lower than in a real gizzard, but still too high to maintain or develop surface polish. If high polish occa- stomach stones sionally seen on sauropodomorph dinosaur gastroliths was indeed caused in a stomach artificial gizzard environment, it implies digestive processes different from those of extant birds and the clast surface development “artificial gizzard”. -

Species in Lake Malawi Dalitso R
The Chambo Restoration Strategic Plan Edited by Moses Banda Daniel Jamu Friday Njaya Maurice Makuwila Alfred Maluwa CHAPTER | Topic i The Chambo Restoration Strategic Plan Proceedings of the national workshop held on 13-16 May 2003 at Boadzulu Lakeshore Resort, Mangochi Edited by Moses Banda Daniel Jamu Friday Njaya Maurice Makuwila Alfred Maluwa 2005 Published by the WorldFish Center PO Box 500 GPO, 10670 Penang, Malaysia Banda, M., D. Jamu, F. Njaya, M. Makuwila and A. Maluwa (eds.) 2005. The Chambo Restoration Strategic Plan. WorldFish Center Conference Proceedings 71, 112 p. Perpustakaan Negara Malaysia. Cataloguing-in-Publication Data The chambo restoration plan / edited by Moses Banda ... [et al.]. ISBN 983-2346-36-3 1. Fisheries --Malawi--Conservation and restoration. 2. Fish-culture--Malawi--Management. I. Banda, Moses. 639.2096897 Cover photos by: C. Béné, R. Brummett and WorldFish photo collection ISBN 983-2346-36-3 WorldFish Center Contribution No. 1740 Printed by Printelligence, Penang, Malaysia. Reference to this publication should be duly acknowledged. The WorldFish Center is one of the 15 international research centers of the Consultative Group on International Agricultural Research (CGIAR) that has initiated the public awareness campaign, Future Harvest. ii WorldFish Center | Biodiversity, Management and Utilization of West African Fishes CHAPTER | Topic iii Contents Foreword v Acknowledgements vi Executive summary vii Introduction viii Official Opening Address by the Secretary for Natural Resources and Environment Affairs, Mr. G.C. Mkondiwa x Section 1: Review of the Chambo fisheries and biology ...................................................................................................... The status of the Chambo in Malawi: Fisheries and biology 1 M.C. Banda, G.Z. Kanyerere and B.B. -

The Evolution Dynamics of the Strigiformes in the Mediterranean Islands with the Description of Aegolius Martae N. Sp
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Institutional Research Information System University of Turin ARTICLE IN PRESS Quaternary International 182 (2008) 80–89 The evolution dynamics of the Strigiformes in the Mediterranean islands with the description of Aegolius martae n. sp. (Aves, Strigidae) Ã Marco Pavia Dipartimento di Scienze della Terra, Museo di Geologia e Paleontologia, Via Valperga Caluso 35, I-10125 Torino, Italy Available online 14 June 2007 Abstract Living and fossil owls (Aves, Strigiformes) constitute an important group for understanding the evolutionary dynamics of birds in island environments. After their different trends in island evolution, the Strigiformes can be seen as a representative of insular adaptations of birds as a whole. In fact they respond quickly to isolation with deep changes in body size, including dwarfism and gigantism, and allometric variations, such as reduction of wings, lengthening of hindlimbs and strengthening of digits and claws. The only exception is the loss of the ability to fly, which has never been recorded in Strigiformes. In this paper I report on all the endemic owls found in Mediterranean Islands, both living and fossil, in order to emphasize trends in insular evolution and the relationships between the different species sharing a certain island. The description of Aegolius martae n. sp. completes the guild of endemic Strigiformes of the early Middle Pleistocene of Sicily and allows to use Sicily as the best example of a biogeographical island type with intermediate characteristics between the oceanic and the continental ones, with the presence of some non-flying mammals, but the lack of terrestrial carnivores. -

Dinosaur (DK Eyewitness Books)
Eyewitness DINOSAUR www.ketabha.org Eyewitness DINOSAUR www.ketabha.org Magnolia flower Armored Polacanthus skin Rock fragment with iridium deposit Corythosaurus Tyrannosaurus coprolite (fossil dropping) Megalosaurus jaw www.ketabha.org Eyewitness Troodon embryo DINOSAUR Megalosaurus tooth Written by DAVID LAMBERT Kentrosaurus www.ketabha.org LONDON, NEW YORK, Ammonite mold MELBOURNE, MUNICH, AND DELHI Ammonite cast Consultant Dr. David Norman Senior editor Rob Houston Editorial assistant Jessamy Wood Managing editors Julie Ferris, Jane Yorke Managing art editor Owen Peyton Jones Art director Martin Wilson Gila monster Associate publisher Andrew Macintyre Picture researcher Louise Thomas Production editor Melissa Latorre Production controller Charlotte Oliver Jacket designers Martin Wilson, Johanna Woolhead Jacket editor Adam Powley DK DELHI Editor Kingshuk Ghoshal Designer Govind Mittal DTP designers Dheeraj Arora, Preetam Singh Project editor Suchismita Banerjee Design manager Romi Chakraborty Troodon Iguanodon hand Production manager Pankaj Sharma Head of publishing Aparna Sharma First published in the United States in 2010 by DK Publishing 375 Hudson Street, New York, New York 10014 Copyright © 2010 Dorling Kindersley Limited, London 10 11 12 13 14 10 9 8 7 6 5 4 3 2 1 175403—12/09 All rights reserved under International and Pan-American Copyright Conventions. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of the copyright owner. Published in Great Britain by Dorling Kindersley Limited. A catalog record for this book is available from the Library of Congress. ISBN 978-0-7566-5810-6 (Hardcover) ISBN 978-0-7566-5811-3 (Library Binding) Color reproduction by MDP, UK, and Colourscan, Singapore Printed and bound by Toppan Printing Co. -

A New Basal Ornithopod Dinosaur from the Lower Cretaceous of China
A new basal ornithopod dinosaur from the Lower Cretaceous of China Yuqing Yang1,2,3, Wenhao Wu4,5, Paul-Emile Dieudonné6 and Pascal Godefroit7 1 College of Resources and Civil Engineering, Northeastern University, Shenyang, Liaoning, China 2 College of Paleontology, Shenyang Normal University, Shenyang, Liaoning, China 3 Key Laboratory for Evolution of Past Life and Change of Environment, Province of Liaoning, Shenyang Normal University, Shenyang, Liaoning, China 4 Key Laboratory for Evolution of Past Life and Environment in Northeast Asia, Ministry of Education, Jilin University, Changchun, Jilin, China 5 Research Center of Paleontology and Stratigraphy, Jilin University, Changchun, Jilin, China 6 Instituto de Investigación en Paleobiología y Geología, CONICET, Universidad Nacional de Río Negro, Rio Negro, Argentina 7 Directorate ‘Earth and History of Life’, Royal Belgian Institute of Natural Sciences, Brussels, Belgium ABSTRACT A new basal ornithopod dinosaur, based on two nearly complete articulated skeletons, is reported from the Lujiatun Beds (Yixian Fm, Lower Cretaceous) of western Liaoning Province (China). Some of the diagnostic features of Changmiania liaoningensis nov. gen., nov. sp. are tentatively interpreted as adaptations to a fossorial behavior, including: fused premaxillae; nasal laterally expanded, overhanging the maxilla; shortened neck formed by only six cervical vertebrae; neural spines of the sacral vertebrae completely fused together, forming a craniocaudally-elongated continuous bar; fused scapulocoracoid with prominent